
Across chemistry’s landscape, zirconium compounds haven’t drawn the same crowds as iron or copper, but their journey—especially in organometallic forms—has impacted coatings, plastics, and electronics. Back in the middle of the twentieth century, industry began pushing for alternatives to toxic and less efficient metallic soaps. Traditional driers used by the paint sector, including lead-based ones, became a concern. Researchers dove into the field, searching for safer, yet robust compounds that could accelerate curing and improve film hardness. Zirconium isooctanoate entered the scene during this push, with its synthesis and optimization influenced by the era’s growing understanding of transition metal carboxylates. Every phase since, from refining ligand structures to tweaking process yields, highlights how chemists chase balance between reactivity, safety, and performance.
Zirconium isooctanoate takes shape as a viscous, yellowish liquid, standing apart from its powdery inorganic cousins. The compound owes much of its character to isooctanoic acid derived from branched hydrocarbons. This material blends into organic media with much less hassle than standard zirconium salts. Paint manufacturers and ink producers both draw from its ease of use. Unlike cobalt driers, which can give color tints or toxic residue, zirconium isooctanoate supports clear finishes and avoids heavy metal issues. The fluidity and stability cut messy work, streamline mixing, and create reliable, consistent outputs—a non-trivial winning trait for technologists working to scale up batches without constant recalibration.
This compound looks like a straw-yellow to amber oily liquid at room temperature. Density tends to range between 0.95 to 1.01 g/cm³. Solubility runs strong in common non-polar and slightly polar solvents, such as mineral spirits, xylene, and toluene. Water doesn’t dissolve it, part of why it remains stable in many mixtures. The molecular structure centers on zirconium atoms coordinated with carboxylate ligands: the isooctanoic acid side chains add bulk and offer organic phase compatibility. The boiling point stays relatively high, keeping evaporation at bay during normal handling. Chemical resilience means it stands up to weak acids or bases, although strong acids or oxidizers can break it down to simpler salts and oxides.
Industry labels typically mark product content in terms of metal percentage (often 15–20% zirconium by weight), acid value, and viscosity. Purity levels can shift based on manufacturer’s synthesis process and post-reaction cleanup, so strict QC remains crucial. Labels highlight UN transport codes, hazard identifiers, and storage recommendations, warning against exposure to high heat or incompatible reactive agents. Documentation should display batch-specific spectroscopic data—usually IR or NMR evidence confirming proper ligand-metal binding—alongside water, acid number, and any prominent impurities. Detailed technical sheets assist industrial buyers in comparing formulations and setting up safe, quality-assured procedures.
Most large-scale production runs mix isooctanoic acid with zirconium compounds such as zirconium oxychloride or basic zirconium carbonate. Heating triggers the acid to displace weakly bound groups, yielding a carboxylate complex. Water formed during the reaction needs to be removed, since lingering moisture can cloud the product or destabilize storage. Manufacturers often refine the mixture by vacuum distillation, stripping away excess acid and side products. Correct stoichiometry controls final metal content and avoids polymeric gel formation. Anyone invested in process technology knows small tweaks, like altering pressure or using recycled solvents, can improve both yield and environmental footprint.
Zirconium isooctanoate stands up to gentle heating and mixing in solvent blends, making it suitable for use in reaction systems or as a catalyst carrier. Its ligands can be exchanged for other carboxylates if a customer requests unique solubility or reactivity perks. In oxidative curing, this compound activates crosslinking sites in drying oils, speeding up the process without heavy metal contamination. Acidic additives or moisture exposure can cause ligand loss and precipitate formation, issues that operators with field experience often solve by maintaining non-aqueous handling lines and monitoring pH levels of the resin or paint blends.
On the global market, zirconium isooctanoate wears a number of monikers: “zirconium octoate” crops up on many product datasheets. The octoate and isooctanoate terms reflect the base carboxylic acid used, though both point back to branched C8 acids. Trade names supplied by chemical giants like Shepherd or OMG Borchers sometimes introduce modifiers indicating concentration, ligand ratios, or absence of specific trace metals (like “lead-free” or “high-purity” series). Knowing these synonyms saves headaches when comparing products across suppliers or verifying compliance for import/export documentation.
Daily industrial use calls for strict adherence to current EH&S guidelines. Though zirconium isooctanoate offers fewer health risks than heavy-metal driers, skin contact or inhalation during blending can still cause irritation. Smart shop floors run engineered ventilation, personal protective gear, and strict waste segregation to keep exposure low. Manufacturers reference OSHA thresholds and GHS labeling, storing the product in sealed drums out of direct sunlight or sources of ignition. Spills spill cleanup teams must keep non-reactive absorbents ready in case containers leak. Documentation on handling, emergency procedures, and material transfers stays available to users at every step to avoid costly health and regulatory oversights.
Zirconium isooctanoate thrives most in paint and coating formulations, where it plays a major part as a drier and curing accelerator. Resin chemists like how it partners with other driers—like cobalt or calcium—taking over some of the harder-to-regulate oxidative steps without turning the film cloudy or brittle. Use also stretches into ink production, rubber vulcanization, and specialty adhesive systems. Wood finishers get a product that dries quickly and offers reliable gloss, without compromising the safety profile. In oil and alkyd-based paints, replacing toxic lead or tin driers creates a healthier, greener end product. Electronic encapsulation work also leans on its thermal stability and moisture resistance.
Today, academic and industrial labs examine how varying ligand structures affect the catalytic role of zirconium isooctanoate. Groups focusing on green chemistry keep searching for raw material sources with lower carbon footprints and milder synthesis conditions. Some work stretches into customizing zirconium-based driers for waterborne systems—a bigger challenge due to inherent water insensitivity. Analytical teams dig into side-product formation and long-term stability, exploring additives that can lengthen shelf life or buffer against decomposition at high temperatures. By fostering collaboration between universities, resin suppliers, and end-users, new uses and tweaks keep emerging.
Compared to cobalt or lead driers, this compound shows much milder toxicity. Published studies point to low acute toxicity in mammals; at standard exposure, lab animals show minimal health impact. Fume inhalation or significant dermal absorption is rare, provided engineering controls remain active. Long-term ecological impact matters more as environmental agencies regulate release of metallic waste. Researchers continue to monitor aquatic toxicity—to both invertebrates and fish—in the event of accidental unlabeled disposal. Today, paint manufacturers share safety data widely, supporting industry-wide moves to safer, less hazardous drier alternatives.
Demand for less toxic, high-performance additives keeps climbing. Regulatory crackdowns on lead and cobalt speed adoption of alternatives. New research targets cost-effective synthesis of green ligands and renewable-solvent blends, aiming for sustainable coatings and inks. Material scientists pin hopes on zirconium complexes for next-gen anti-corrosion primers and specialty polymers tailored for electronics. As public awareness about chemical safety and environmental impact increases, suppliers who prioritize life cycle analysis, transparency, and continual improvement stand to gain the most. In the years ahead, advancing formulation science, improved metal recycling, and finer process control promise to carve out a strong role for zirconium isooctanoate across emerging industrial challenges.
People working in the coatings and plastics industry often handle chemicals with complicated names, but a lot of those chemicals play basic, important roles that keep products working as customers expect. Zirconium isooctanoate, for example, isn’t a big headline-maker, yet it’s an ingredient that quietly supports a surprising number of modern industries.
Paints and varnishes need to dry in a way that helps them last. Anyone who’s painted a fence or refinished a table has probably noticed: some paints dry harder, tougher, and smoother than others. Zirconium isooctanoate works as a drier in oil-based paints. By speeding up how paint films cure, it helps coatings turn from a sticky layer to a usable, water-resistant surface. This is less about simple “speed” and more about the finished product—hard films last longer on everything from doors to playground slides.
Manufacturing runs on efficiency. A dried paint film too slow off the line can slow down a whole factory. I’ve seen production lines where even a few minutes’ delay triggers a pileup—goods start collecting dust, machines go idle, and costs climb. Using a drying agent like zirconium isooctanoate means companies can keep paint and ink production moving. This leads to less waste, better worker morale, and products that don’t sit around gathering dust, literally or figuratively.
Many traditional driers, like cobalt compounds, bring their own headaches. They can trigger concerns about toxicity and waste. Workplaces take safety more seriously now, and people want fewer heavy metals in daily life. Zirconium isooctanoate brings much lower toxicity compared to cobalt. This means safer working conditions and end-use products—an important shift for creators and consumers. Some governments have even set new restrictions on toxic driers, pushing demand toward chemicals like zirconium isooctanoate.
Zirconium isn’t just for paints. Printers use it in inks, giving quick drying ability to everything from magazines to packaging. Plastics makers also turn to zirconium isooctanoate for cross-linking polymers. Well-cured resins hold color and shape better—which matters to someone buying a colored water bottle or sturdy food container.
Safer, more environmentally friendly ingredients need support from both regulators and industry leaders. Investing in research will mean even cleaner options down the road. In my own work with manufacturers, I’ve noticed how companies now prioritize not just technical performance but the safety labels on every drum and pail. I encourage companies to keep pushing green chemistry: the less toxic the production plant, the better everyone feels—on the job and at home.
So, while zirconium isooctanoate won’t attract much attention at the checkout aisle, it remains a backbone for reliable, safer products—coatings, plastics, and inks among them. It’s proof that the building blocks of modern life matter just as much as the bold colors and hard finishes they help create.
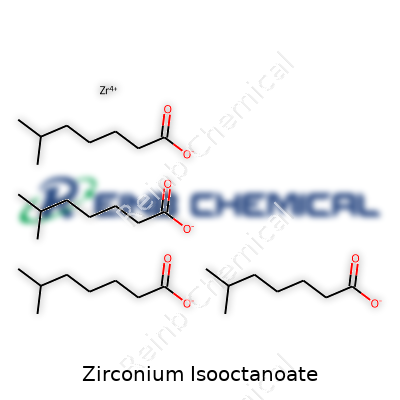
The chemical formula for Zirconium Isooctanoate reads as Zr(C8H15O2)4. In plainer terms, a single zirconium atom connects with four isooctanoate groups. Each isooctanoate group consists of eight carbon atoms, fifteen hydrogens, and two oxygens, commonly called isooctanoic acid before it latches onto the metal. Factor these together, and you get a complex that looks simple on paper but plays a big part across many industrial corners.
Back during my short-lived stint as a lab assistant at a metals precursor startup, I helped measure out bottles of zirconium isooctanoate, usually in clear, oily liquid form. This compound plays a major role as a drier in alkyd-based paints—speeding up the hardening process for coatings on bridges, shipping containers, and sometimes the metal playground equipment standing in the park where my neighbors’ kids roughhouse.
Zirconium isooctanoate works as a catalyst, helping oxygen "bite" into unsaturated fatty acids in paint. This sets off a reaction that turns soft paints into tough, weather-resistant coatings. Unlike old-fashioned driers, it doesn’t come with the health baggage that cobalt driers carry, so coatings scientists and job-site painters feel a little more comfortable breathing around it.
Most people don’t get excited thinking about “metal carboxylates,” but behind every practical product, someone has to reckon with environmental weight. For large-scale paint manufacturers, using zirconium isooctanoate helps meet stricter environmental standards. Cobalt and lead-based driers raise red flags for toxicity and bioaccumulation. Zirconium, by contrast, doesn’t belong to the list of toxins that turn up in landfill soil tests, and while not edible, exposure to trace amounts doesn’t carry major risks. Experience shows that less hazardous stuff, even if a bit pricier, wins long-term as regulations lock down the use of heavy metals.
Getting high-purity Zirconium Isooctanoate costs more than many expect. A few years ago, global supply chain hiccups drove up prices, hitting paint and coating manufacturers particularly hard. Some tried to blend their own, using technical-grade isooctanoic acid, but product quality took a noticeable hit. If you try making high-quality paints or inks without pure sources, the end result might lose gloss or cure unevenly—something I saw firsthand during quality-control days at the plant.
The cost pressure has nudged researchers to search for alternatives or to reuse and recycle more material at production sites. Chemical engineers are tinkering with ways to reclaim unused drier from the rinse tanks and distill it for a second run. Solutions like that stem from teams grounded in shop-floor realities who see every lost drop as wasted potential and higher costs on tomorrow’s balance sheet.
Zirconium isooctanoate doesn’t just make paint dry quicker; it lifts product safety benchmarks. Its chemical profile, Zr(C8H15O2)4, supports coatings that last longer, chalk less, and pose fewer health risks. For end-users, this means roofs that look fresh for years, fewer break-downs on machinery, and jobsites that don’t need warning labels on every barrel. In the bigger picture, the right chemical choices support healthier workers and a cleaner footprint—something every industry could stand to care about more.
Zirconium isooctanoate helps dry paints, inks, and varnishes faster and more evenly. Its strength comes from how well it triggers chemical reactions, making it valuable in factories and workshops. Many coatings on furniture or car parts last longer because of this compound.
People often ask if zirconium isooctanoate is safe to handle. Personally, I wouldn’t treat anything from the world of chemical catalysts lightly. The safety data from several manufacturers warns about skin, eye, and respiratory irritation. Even if a product gets labeled as a “low hazard,” I remember stories from the shop floor where someone got lazy with gloves and goggles, ending up with painfully red skin for days. Small mistakes can pile up quickly.
A close look at the safety sheets tells you it’s not a chemical to splash around carelessly. Contact with skin or eyes causes real discomfort, sometimes burns. Inhaling vapors or dust doesn’t do your lungs any good either. There isn’t enough proof out there saying it can cause long-term illness, but good habits now prevent regret later on. Choosing to skip gloves or cheap out on proper ventilation looks tempting until you’re coughing or itching for days.
Scientific data from groups like the European Chemicals Agency flags zirconium isooctanoate as irritating. A study in a coating factory found that workers who didn’t use gloves or masks reported up to three times more skin rashes. The Centers for Disease Control and Prevention lists zirconium compounds among substances needing eye and skin protection, even if toxicity stays low. The lesson: you won't drop instantly from exposure, but repeated or careless mishandling builds up risk.
I’ve seen some workers rely too much on the idea that it’s less dangerous than older, toxic driers like cobalt. True, it’s less harsh, but that doesn’t make it safe in everyday use. Responsible handling keeps small problems from exploding into bigger ones—especially in places where spills or splashes aren’t that rare.
Factories and studios have a few simple moves to cut risks. Gloves and goggles don’t just sit in the safety locker—make them as normal as putting on a uniform. Strong ventilation, either natural or with a fan, clears out any fumes before they ever reach your lungs. Training goes beyond a quick video; a real-life safety drill once or twice a year reminds everyone why it matters.
Managers sometimes resist spending extra on training or gear. But when you count lost days, workers’ comp claims, or the cost of an accident, prevention pays for itself many times over. Bringing in health professionals for expert advice shines a light on risks people forget after years on the job.
Nobody expects accidents—then someone turns away from the bench and gets a face full of chemicals. I’ve worked in places where the difference between a good day and a trip to the clinic is wearing gloves or remembering to wash up right after work. No one brags about being careless with something that can burn eyes or skin. Handling zirconium isooctanoate with respect protects everyone, from the new recruit to folks with decades of experience. The chemical can help build quality products, but only if people use it smarter, not just faster. Trust habits proven to work, and keep eyes open for new ways to build a safer workplace every day.
Zirconium isooctanoate works quietly in a range of chemical processes, often involved behind the scenes in coatings, paints, and even plastics. It looks tame on the surface, but it reacts to some simple things in ways that can ruin a batch or, worse, harm someone. From years of working around specialty chemicals, ignoring the fundamentals of storage turns an expensive investment into a costly hazard.
Storing this material in tightly sealed metal or high-quality plastic drums prevents exposure to oxygen and moisture. The biggest practical problem comes from leaks or vapor escape. Exposure to air can trigger oxidation and hydrolysis, leading to the formation of insoluble precipitation. That means ruined product, lost money, and calls to the waste disposal company.
Corrosive reactions sometimes chew through lesser containers over time. High-density polyethylene (HDPE) drums or properly lined steel containers keep the material inside and out of trouble. Avoid glass, since it can’t stand up to mechanical impacts and can shatter, spilling contents across concrete floors.
Temperature makes a difference. Stores have shown that direct heat shortens shelf life, causing the liquid to become thick or develop odors. Rooms sitting at 20–25°C (68–77°F) avoid these risks: cool enough for safety but not so cold that things turn solid. Warehouse managers should monitor for hot patches near machinery or sunlight streaks that sneak through windows.
Moisture, even in small quantities, kicks off unwanted reactions. Desiccants or dehumidifiers come in handy, especially in coastal regions or during humid summers. Storing drums on pallets, never directly on concrete, helps air circulate under the container and reduces corrosion risk from invisible leaks or sweating floors.
One gallon of spilled or improperly sealed product attracts dust, debris, and even rodent visits—each a possible source of contamination. Use good discipline: open containers only when needed, close them firmly, and never reuse sealing gaskets or stoppers once damaged. Chemical cross-contamination doesn’t just degrade performance; it often triggers broader safety problems.
Disposable lab coats and gloves protect staff, but clean scoopers and funnels matter just as much. Never let residue accumulate on drum exteriors. Staff with years on the job know that sloppy storage habits almost always lead to emergency cleanups or hazardous exposures.
Regulatory agencies across North America, Europe, and Asia make strict demands about how to handle and store metal carboxylates. Regular inspection checks, with logs accessible by supervisors, support both operational safety and regulatory compliance. Up-to-date labels with lot numbers, hazard symbols, and expiration dates make a difference during audits and daily checks.
In my lab days, a forgotten or misfiled label could mean lost traceability. So double-checking record-keeping isn’t just about bureaucracy; it’s about safety and accountability. Digital inventory systems and scan-ready barcodes bring accuracy and convenience without slowing the pace on busy days.
No storage plan counts as complete unless it looks ahead to accidental spills, fires, and leaks. Spill kits with absorbent materials, chemical-resistant gloves, and instructions hang near storage bays. Fire extinguishers rated for liquid chemicals sit within reach. Emergency procedures feel pointless—until the one day they save lives and keep insurance claims at bay.
People matter more than protocols. Every person working with hazardous chemicals should learn safe storage principles, not through manuals alone, but with hands-on walk-throughs and routine drills. Peer-to-peer instruction, especially from someone who has seen what goes wrong with shortcuts, keeps everyone alert and prepared for real-world problems.
Zirconium isooctanoate doesn’t grab headlines, but chemists and manufacturers recognize the value it brings, particularly in coatings and paint driers. Handling and packaging this specialty chemical present challenges that most people don’t think about until there’s a leak, a delay, or some mysterious powder coating your loading dock.
Most shipments show up in drums. Not the kind you hear in a parade but hefty steel or plastic barrels, usually at 200 kilograms per drum. Thousands of factories across Asia and Europe rely on these containers. Anyone dealing with reactive or slightly volatile substances understands the need to keep moisture and air out. Screw caps and security seals aren’t just for show — they reduce spoilage, contamination, and safety risks.
Some large buyers prefer intermediate bulk containers (IBCs) for quantities above one ton. You’ll find these square tanks with tap-systems stacked in industrial yards. For small labs, samples sometimes get shipped in metal cans or HDPE bottles, each carefully labeled and tightly closed. A lot of suppliers offer special packaging for urgent or research orders, especially when customers want to hedge against cross-contamination.
Temperature swings raise their own problems. One summer, a batch sat inside a steel drum near a hot delivery dock for days. The cap bulged and the liquid kicked up a sharp smell. Luckily, just some product lost. It’s not only about meeting legal safety codes; packaging impacts bottom line and quality on nearly every delivery run.
Compliance means practical steps, not just paperwork. European REACH rules and similar standards in the US or Japan require manufacturers to track batch numbers straight through to end-users. Clear hazard labels, UN numbers, and detailed certificates of analysis travel with every shipment. A QR code or RFID tag helps customers log and trace the shipment, making recalls or audits a lot less painful.
Every drum, pail, or bottle leaves a footprint. Old-fashioned suppliers sent back empties or arranged for cleaning at distribution hubs. Today, sustainability drives big decisions. Some producers partner with closed-loop recycling outfits. These days, caps, liners, and even labels come in compostable or recycled plastics. Buyers, especially in Europe, now ask more questions about how suppliers take care of empty containers.
There’s also a growing expectation for digital tracking. Several companies put a GPS chip in bulk shipments, giving factories a better handle on timing and safety during transport. Not every player has caught up, but the leaders set the standards others have to follow.
The future looks interesting. Researchers are piloting drums with built-in sensors that track temperature and pressure in real time. Imagine an alert going off at the first sign of a leak or overheating, instead of hoping someone spots it before damage spreads. This isn’t sci-fi; a few German sites tested prototypes last year.
Supplying chemicals like zirconium isooctanoate isn’t just about the liquid in the drum. The story of supply, from drum to door, shapes cost, safety, and long-term environmental impact. Every improvement—smarter seals, real-time tracking, less plastic in packaging—brings the industry closer to the transparent and responsible workflows customers now expect.
| Names | |
| Preferred IUPAC name | 2-ethylhexanoatozirconium(4+) |
| Other names |
Zirconium 2-ethylhexanoate Zirconium octoate Zirconium(4+) 2-ethylhexanoate Zirconium neodecanoate |
| Pronunciation | /zɜrˈkoʊniəm aɪsəʊˈɒktəˌnoʊeɪt/ |
| Identifiers | |
| CAS Number | 22464-99-9 |
| Beilstein Reference | 2124984 |
| ChEBI | CHEBI:53262 |
| ChEMBL | CHEMBL4297807 |
| ChemSpider | 19821024 |
| DrugBank | DB15959 |
| ECHA InfoCard | 03-2119948574-48-0000 |
| EC Number | 242-016-8 |
| Gmelin Reference | 80541 |
| KEGG | C17257 |
| MeSH | D017936 |
| PubChem CID | 166873 |
| RTECS number | ZR1750000 |
| UNII | 55MAD25A2J |
| UN number | UN#1438 |
| CompTox Dashboard (EPA) | DTXSID2022237 |
| Properties | |
| Chemical formula | C24H44O4Zr |
| Molar mass | 612.1 g/mol |
| Appearance | Clear pale yellow liquid |
| Odor | Characteristic |
| Density | 1.04 g/cm3 |
| Solubility in water | Insoluble in water |
| log P | 3.6 |
| Vapor pressure | Negligible |
| Acidity (pKa) | 13.15 |
| Basicity (pKb) | pKb ≈ 9.8 |
| Refractive index (nD) | 1.488 |
| Viscosity | 300 - 600 cP |
| Dipole moment | 1.82 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 357.6 J·mol⁻¹·K⁻¹ |
| Pharmacology | |
| ATC code | V09AX01 |
| Hazards | |
| GHS labelling | GHS02, GHS07 |
| Pictograms | `GHS07` |
| Signal word | Warning |
| Hazard statements | H315: Causes skin irritation. H319: Causes serious eye irritation. |
| Precautionary statements | P261, P264, P271, P273, P280, P302+P352, P305+P351+P338, P312, P332+P313, P337+P313, P362+P364 |
| NFPA 704 (fire diamond) | 1-3-1-W |
| Flash point | 102°C |
| Lethal dose or concentration | LD50 (oral, rat) > 5000 mg/kg |
| LD50 (median dose) | LD50 (median dose): >5,000 mg/kg (rat, oral) |
| NIOSH | Not Established |
| PEL (Permissible) | PEL (Permissible Exposure Limit) for Zirconium Isooctanoate: 5 mg/m³ (as Zr, OSHA PEL) |
| REL (Recommended) | 10000 mg/kg |
| IDLH (Immediate danger) | Not established |
| Related compounds | |
| Related compounds |
Zirconium 2-ethylhexanoate Zirconium octoate Zirconium neodecanoate Zirconium naphthenate Zirconium acetate Zirconium propionate Zirconium butyrate |