
Zinc isooctanoate enters the story of modern chemistry through the constant search for materials that strike a balance between reactivity and safety. In the early part of the twentieth century, researchers wanted metal carboxylates with low volatility and stable performance for industrial uses. The broad development of metal soaps began around the time paint and rubber industries demanded additives that improved weather resistance. Zinc isooctanoate found its place as chemists identified the advantages of branched-chain carboxylic acids, seeking improvements over traditional metal stearates and palmitates. By focusing on isooctanoic acid—a branched, medium-chain acid—developers opened a pathway for improved solubility in organic media while retaining the beneficial characteristics of zinc. This transition wasn't immediate; bench-testing and pilot-scale trials throughout the 1950s and 1960s eventually brought this material to both paint driers and stabilizer blends. My first brush with zinc isooctanoate came working in a coating lab, trying to troubleshoot drying defects in alkyd paints, and I learned quickly how minor differences in metal carboxylates can tip the scale between smooth and tacky finishes. People who work in paints or plastics tend to remember when the right additive made their batch click.
People in industry rely on zinc isooctanoate for more than just drying. Every barrel or drum it comes in contains a colorless or faintly yellowish liquid or soft solid, depending on the precise formulation and temperature. It’s often found dissolved in white spirit, mineral oil, or a similar carrier for easier handling. Supply chains feed it to manufacturers who work on paints, coatings, plastics, and rubber. What keeps it in use is its ability to interact kindly with other components in a formulation. It doesn’t cloud clear coatings unless over-applied, and it mixes without fuss into most standard resins. Its odor—a slightly fatty, waxy aroma with a metallic edge—sticks around in plant hallways, unmistakable once you know it. Formulators usually opt for zinc isooctanoate grade tailored for activity, solubility, and metal content, as these parameters help dial in compounding accuracy.
Technical datasheets describe zinc isooctanoate as a coordination compound between divalent zinc cations and two isooctanoate anions. Its molecular weight typically sits around 368 g/mol, but the figure can slide depending on water, solvent, or other minor components tied up in the material as received. In pure form, it melts at roughly 36–46°C and starts decomposing beyond 200°C. Limited water solubility characterizes most of these metal soaps, so they separate from aqueous phases easily but disperse generously in non-polar or weakly polar solvents. The compound’s density, usually between 1.05 and 1.15 g/cm³, allows it to blend easily with typical organic liquids without severe settling. Its chemical and thermal stability support industrial processes like extrusion or curing cycles reaching moderate heat. Its amphiphilic nature—both fatty and metallic—gives it an edge in compatibilizing resins and waxes with mineral oil or organic thinners.
Good production practice and regulatory needs call for standardized labeling on every shipment. A typical label might show the zinc content, often as a percentage or weight per volume (e.g., 10% Zn by mass). Look for complete information on acid number, solvent carrier, free acid content, and, if present, other stabilizing additives, as minor details sometimes affect process compatibility. Batch records might also mention melt point ranges and typical pH of a 10% suspension in methanol or hexane. For labeling in international shipping or regulated markets, suppliers add GHS hazard symbols, proper shipping names (usually “Zinc Compound, n.o.s.”), and reference numbers from CAS (15770-94-8) or EINECS. One tip: before unloading, always cross-check with a fresh lab analysis for metal content—a practice that saved one coating line I oversaw from days of troubleshooting an underperforming batch.
Production of zinc isooctanoate most often kicks off with the reaction of zinc oxide or zinc carbonate with isooctanoic acid. Many plants mix these under an inert or nitrogen blanket to dodge unwanted oxidation or water pickup. The acid reacts with solid zinc sources in a stirred reactor, gently heated—temperatures over 100°C speed up the reaction but risk minor byproduct formation. Stirring continues until CO2 evolution (for carbonate methods) or water formation tapers off, signaling the endpoint. Careful distillation or vacuum stripping removes excess acid, water, and low-boiling fractions. Technicians monitor the batch using titration for free acid and gravimetry or ICP for zinc content. Large-scale operations sometimes add a thin film or wiped film evaporator stage to polish the product. I once visited a plant where process engineers built in a buffer tank, giving them leeway to fine-tune the zinc ratio on the fly—a trick worth remembering if processing volumes fluctuate.
In industrial use, zinc isooctanoate’s reactivity stays centered on its ability to exchange carboxylate ligands and to mediate polymerization or crosslinking processes. It’s not easily reduced or oxidized, which helps in harsh curing ovens or under variable storage conditions. The compound can react with hydrophilic acids or chelators, so accidental contact with water-soluble chelating agents or phosphates might pull out the zinc and create hazes or precipitates. Manufacturers sometimes modify it by mixing with other metal carboxylates—cobalt or calcium, for example—creating synergistic blends for faster drying in coatings or improved stability in plasticizers. Other industries convert zinc isooctanoate into downstream catalysts or stabilizers using transesterification or saponification reactions, broadening its utility beyond direct addition. Practical knowledge of its chemical landscape prevents headaches down the line—twice in my experience, careless blending with incompatible surfactants led to complete product failures.
Depending on the supplier, you’ll see it called zinc isooctanoate, zinc 2-ethylhexanoate, zinc octoate, or zinc neo-octanoate. The term “octoate d’isooctyle de zinc” pops up in French technical literature, while the German market sometimes uses “Zink-2-ethylhexanoat.” Each name refers to the same metal salt of 2-ethylhexanoic acid, which is itself an isooctanoic acid variant. Commercial designations may list concentrations—for example, “Zinc 2EH 8% in mineral spirits.” Always double-check labels or specifications from new vendors because regional differences in nomenclature sometimes sow confusion in ordering or regulatory declarations.
Zinc isooctanoate checks off as relatively safe compared to heavy-metal alternatives, but that doesn’t mean casual handling works in a modern plant. Both zinc and isooctanoic acid call for standard chemical hygiene—avoiding inhalation and prolonged skin contact, using gloves and eye protection, and working in ventilated spaces. Most material safety data sheets highlight mild irritation potential for skin or eyes, along with flammable carrier solvents where used. Safe storage means cool, sheltered spaces and tight caps to prevent moisture ingress or color change. Clean-up of spills uses absorbent granules—avoid water flushes, as the compound doesn’t disperse well and may leave slippery residues. Strict attention to GHS or OSHA standards, including proper secondary containment and documentation, reduces risk of reportable spills. For years, routine safety drills in my teams caught minor errors before they turned into costly incidents.
Paint and coatings industries get the lion’s share of benefit from zinc isooctanoate; it catalyzes the oxidative drying of alkyds and oil-based formulations, pulling together film formation at workable speeds. In plastics, it stabilizes PVC against dehydrochlorination, maintaining clarity and mechanical toughness under heat or sunlight. Rubber compounds gain from its use as a processing lubricant and release agent, streamlining molding and improving product appearance. Printing inks, adhesives, sealants, and even cosmetics look to zinc isooctanoate for controlled metal delivery in non-polar systems. More niche uses include as a plasticizer or as a metallic soap catalyst in specialty polyester manufacturing. I remember troubleshooting at an injection molding plant where switching from barium to zinc isooctanoate cut odor complaints drastically, with no hit to throughput or surface quality.
Active research pushes zinc isooctanoate into new spaces: high-performance coatings for marine and automotive uses, next-generation plastic stabilizers, and green chemistry alternatives to lead-based driers. Lab teams work to lower VOC content in commercial blends and to tune the ligand environment for improved weathering resistance. Some studies dive into nano-scale dispersion, coaxing out even more effectiveness per gram delivered. Universities and industrial consortia test variations on structure—switching out chain lengths, branching, or substituents—to squeeze out higher efficiency or reduced migration. I’ve followed teams tackling the biobased feedstock side, aiming for renewable isooctanoic acid sources rather than fossil-fuel derivatives—a tough challenge but one with real environmental upside.
Zinc compounds tend to carry low mammalian toxicity when used as directed, and zinc isooctanoate does not buck this trend. Research across regulatory agencies points to mild skin or eye irritation as the most common risk, with significant acute effects rare outside of accidental ingestion or chronic overexposure. Studies in aquatic systems, though, spotlight zinc’s persistence—high discharge levels can harm fish or invertebrates—so wastewater controls matter. Chronic toxicity studies in rodents reported little bioaccumulation or systemic effects at doses far above workplace exposure limits. Scientists continue to monitor for long-term or subtle endocrine effects, especially as regulatory thresholds for metallic soaps evolve. Keeping up with this literature shapes responsible plant safety and community engagement strategies.
Demand for zinc isooctanoate looks steady, maybe even rising as industries seek to eliminate toxic metal additives and meet ever-tightening environmental guidelines. Growth areas sit in low-VOC coatings, sustainable plastics, and specialist lubricants that take advantage of its unique blend of stability and compatibility. Labs keep aiming for improved bio-based synthetics, lower energy production, and reduced waste side streams. Market shifts toward circular economy practices favor compounds that can be recycled or safely handled at end-of-life. For the next decade, success will follow those who optimize both performance and environmental profile—a goal that keeps both researchers and plant engineers busy, and from my seat, one worth pursuing.
Zinc isooctanoate doesn’t get a lot of headlines, but this compound pulls real weight behind the scenes. I’ve noticed people rarely talk about what goes into the paint on our walls, or the plastic that keeps food fresh. Zinc isooctanoate works as a catalyst, especially in products based on polyurethane. Manufacturers put it to work in flexible foams, coatings, and adhesives. Restaurants depend on polymer flooring that stays tough and easy to clean; cars roll off the line with seats made more resilient through effective zinc-based catalysts.
People tend to overlook how catalysts like this speed up chemical reactions without lingering in the end product. In factory settings, using zinc isooctanoate streamlines the making of everything from insulation panels to synthetic leather. Faster production means lower costs and less energy wasted. That matters a lot when energy bills keep climbing. And, for anyone building or renovating, better use of catalysts means doors, flooring, and even mattresses can be manufactured with greater consistency and less waste.
Each time I pick up a polyurethane item, I remember hearing questions about chemicals and safety. Most data shows zinc isooctanoate doesn’t build up in our bodies or escape into food, when handled right. Health agencies require close monitoring to keep exposure within safe limits. That matters because workers face constant contact, and any slip in safety practices could raise risks of skin irritation or other health problems. Factories using strict controls and real-time monitoring can reduce these risks, and those lessons often trickle down into small workshops and even labs at local colleges.
Responsible sourcing and clear labeling protect workers and consumers. Firms using zinc isooctanoate in coatings for food packaging or child products must track purity and follow strict process controls. In my own experience working alongside industrial chemists, I’ve seen strong systems in place for documentation and worker training. This kind of vigilance means fewer accidental exposures, and more trust from end users who worry about what touches their children’s toys or restaurant food containers.
Though zinc isooctanoate streamlines finishing steps in many industries, questions about environmental effects still pop up. Zinc-based compounds don’t linger in the environment like some metals, but wastewater from factories can carry trace amounts downstream. Real progress happens when companies invest in tight recycling and filtration. In regions facing water supply concerns, these setups protect not just factory workers but also everyone who relies on clean water further down the line.
Research into alternatives keeps growing. Some companies now look to bio-based catalysts or blend zinc isooctanoate with stabilizers that limit emissions. In places where regulations tighten every few years, this push for cleaner chemistry shapes whole product lines. I’ve met small manufacturers experimenting with lower-dose catalysts and automated batch systems, which reduce spills and cut raw material use. That kind of practical problem-solving gives me hope for both safer workplaces and less impact on the planet.
Zinc isooctanoate comes up often in manufacturing, coatings, and some chemical processing tasks. It plays a useful role in curing rubber and as a drier in paints. But working with this material asks for respect. Over the years, I’ve learned that dismissing chemical risks, big-name or not, opens the door to unwanted health problems or costly mistakes. Taking a few moments to consider safe handling helps protect everyone in the workspace.
I’ve seen far too many people skip gloves just because a product doesn’t cause instant burns. Zinc isooctanoate can irritate skin and, more dangerously, trigger eye discomfort if it gets in by accident. Wearing butyl or nitrile gloves won’t slow you down; they keep that stuff from settling into your hands or under your fingernails. Splash goggles or safety glasses fit snug over your eyes, blocking wayward drops or dust. Simple habits—like always wearing gloves and not rubbing your eyes mid-task—reduce the odds of an incident.
The fumes can sneak up quietly if there’s poor ventilation. My old factory ran into headaches—literally—when solvent vapors built up in a back storage room. It pays to work in an area with open windows or, better yet, proper exhaust ventilation. Respirators step in only if local air flow can’t keep up with vapor levels, so knowing your air quality from regular monitoring matters. Following advice from OSHA and NIOSH about air changes per hour makes sure nobody risks lung problems.
I heard a story from a friend in paint production: Two drums got stored too close together, and cross-contamination caused a spill. It’s a headache to clean and, worse, some mixtures spit out dangerous vapors. Keeping zinc isooctanoate away from strong acids and oxidizers cuts back on fire risk. Label everything clearly and double-check before pouring or mixing. If you spot a spill, handle it while wearing protective gear. Use absorbent pads, not towels, then seal the waste tightly before tossing. Plus, keeping an eye on the storage temperature helps—no need for things to heat up and react in the dark.
Workplaces improve when everyone takes service areas and break rooms seriously. It’s common to skip showers after a shift, but washing skin and changing clothes is key to keeping any chemical residue out of your car and home. For unfamiliar products, safety data sheets hold a lot of answers—what to do after a splash or spill, what first aid to use, and even what long-term exposure can mean for your health. Reading those sheets once, then keeping them close at hand, makes response times quicker and more effective.
Many places benefit from short, regular training refreshers. Walk through real-life spills, review the gear, practice using eyewash stations together. Teams learn best when talking openly about near misses, so share stories and lessons without finger-pointing. Investing in reliable gloves, goggles, and good ventilation repays itself by reducing downtime, medical expenses, and insurance claims. Every extra safeguard reinforces a culture where safety isn’t paperwork—it’s just the way good work gets done. With zinc isooctanoate or anything stronger, that approach pays off.
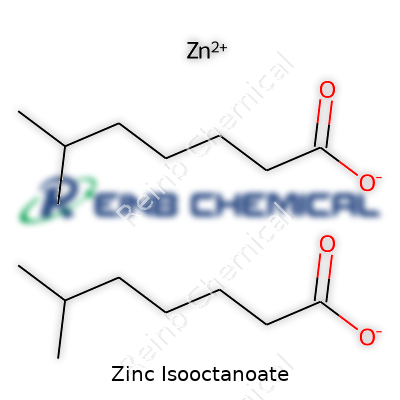
Zinc isooctanoate brings together two chemicals that work well in several industrial uses. The chemical formula of zinc isooctanoate is Zn(C8H15O2)2. This combines zinc with two molecules of isooctanoic acid, also called 2-ethylhexanoic acid or octanoic acid. Each molecule of isooctanoic acid features eight carbon atoms, fifteen hydrogens, and two oxygens. When paired with zinc, the resulting molecule helps power certain industrial reactions and product formulations in a way pure zinc can’t.
Zinc isooctanoate turns up in paints, coatings, PVC stabilizers, and lubricants. You might not think about it, but that smooth look on metal parts sometimes owes its life span to this little molecule. In paint, zinc isooctanoate helps produce a fast hardening of oils and resin, especially in alkyd paints. This means shorter drying times for painted surfaces and less risk of dust sticking on the wet surface. In plastics, the substance offers stability against heat and ultraviolet rays, which can be a big deal for keeping garden furniture or window frames from breaking down in sunlight.
When I managed a construction project, I learned to appreciate products with additives like zinc isooctanoate because they extended the lifespan of the paint jobs on metal fences and structures, cutting down maintenance costs. Both home renovators and industry folks look for these benefits, even if they rarely read what goes into the paint cans or plastic pellets.
Some old-school stabilizers, especially those containing lead, have dropped from use for health and environmental reasons. Zinc isooctanoate offers a safer alternative in many of the same uses, especially for PVC. I read reports from polymer industry watchdogs that highlight fewer risks with zinc-based stabilizers. That means safer working conditions and less risk when plastics eventually get recycled or tossed. Regulatory shifts back this track, since government bodies in the U.S., EU, and elsewhere restrict heavy metal stabilizers, urging the use of compounds with better health profiles.
The molecule isn’t just benign, though. Zinc compounds, even those considered relatively safe, have to be managed in a way that avoids buildup in soils and water. Once, in a small urban gardening project, I noticed the difference in plant health in soil with high zinc content. Too much zinc, even from supposedly safe additives, can strain ecosystems. This experience pushes me to support more exact tracking and closed manufacturing loops wherever possible, so less ends up in rivers, soil, or the wider food chain.
Chemical production leaves traces, and the industry around zinc isooctanoate isn’t immune. Hurdles remain in efforts to cut down waste or ensure recoverability. Some producers now design ways to reclaim and reuse stabilizers, either by engineering materials to be more easily separated or in chemical recycling. This creates pathways for better stewardship, even in processes people rarely see.
Zinc isooctanoate reflects the kind of molecule where smarter use and clear understanding bring practical advances. The move from lead and cadmium compounds over the past few decades demonstrates that change is possible. Instead of sticking to additives that merely work “well enough,” I think more knowledge, tighter environmental controls, and transparent labeling can help both professionals and everyday users get the products they need without as many trade-offs down the road. Knowing the formula—Zn(C8H15O2)2—opens the door to smarter questions and even better answers next time you choose a product off a shelf.
Zinc isooctanoate doesn’t show up on most grocery lists or in backyard sheds, but those who handle chemicals in manufacturing, research, or paint production know it well. Storing this compound correctly isn’t just a matter of ticking regulatory boxes—real safety and product performance depend on it. I’ve seen firsthand what happens when a specialty chemical sits too long on a shelf beside the wrong material or in a humid corner of the warehouse. More often than not, you learn the hard way just how quickly things go south.
Zinc isooctanoate will react to air, heat, or moisture over time. All it takes is a careless storage spot—maybe near a leaky window or close to a heat source—to ruin a batch or spark an emergency. The best way to avoid surprises is to keep it in a spot protected from direct sunlight. Don’t just shove it onto any shelf or leave it close to radiators. A cool, dry, well-ventilated area does more than preserve the chemical; it cuts the risk of fumes or degradation that can put workers and equipment at risk.
Any container holding zinc isooctanoate should get special attention, too. Old, beat-up drums let in air or leak, so I always check for tight, corrosion-proof lids. Unapproved containers, or those made with reactive metals, bring their own problems—corrosion, contamination, and, in the worst-case, fire risks if vapors escape or mix with incompatible materials.
The trickiest part comes from mixing up containers. Someone might set a half-drummed keg of ethyl acetate next to the zinc isooctanoate, thinking, “One row for all the organics, problem solved.” Except it isn’t—certain combinations don’t play well, and missing a label or an obscured hazard sign leads to real headaches. warehouse supervisors who skip a weekly walk-through sometimes discover a puddle or odd smell days too late. I get why people rush, but it takes minutes to check labels for each item, keep them in clear sight, and ensure chemicals of the same hazard class group together.
A written safety data sheet doesn’t mean much if people treat it like a formality. In every plant I’ve worked in, the best result came from simple, recurring reminders: dry place, sealed container, away from acids, bases, peroxides, or strong oxidizers. New hires and seasoned staff need more than rules—they need routine walk-throughs to prove practices match what’s on paper. Practical skills save money, time, and injuries. Spills from improper storage cost thousands, not to mention downtime and spoilage.
Automated climate controls and regular safety inspections cost less than damaged product or regulatory fines. For companies working with small batches, even a high-quality cabinet with temperature and humidity monitoring makes a difference. If precise records back up routine checks, managers can act fast—and auditors get proof of compliance. Safe storage of zinc isooctanoate marks a culture of care from the top down, showing workers and clients the company values health and safety as much as production.
To sum up the real-world lesson: good storage habits protect more than raw goods—they keep teams safe, prevent fines, and preserve quality. A few simple steps, kept up consistently, pay off every single time.
Zinc isooctanoate pops up in several industries, especially in coatings, plastics, and rubber manufacturing. Its role as a catalyst and a stabilizer helps in shaping modern products that people rely on daily. Factories prize it for speeding up chemical reactions while keeping costs under control. Yet, few everyday consumers recognize the name or consider what happens to it down the line. This unfamiliarity sometimes leads to a blind spot in both safety awareness and environmental responsibility.
Having spent years teaching high school chemistry, I've watched students misunderstand the difference between elemental zinc, which the body does need in small amounts, and zinc compounds, which can behave unpredictably in biological systems. Zinc isooctanoate doesn’t belong anywhere near a dinner plate. In workplaces, inhaling the dust or fumes could cause sore throats or respiratory trouble, especially in poorly ventilated spaces. Repeated skin contact might also trigger irritation.
It's not likely to cause widespread havoc with casual exposure, but a chronic disregard for proper gloves, masks, or extraction systems leads to stories of rashes, coughing, or worse over time. Studies published by organizations like the European Chemicals Agency underline that it's not acutely toxic in low doses. Chronic exposure, though, always increases the stakes.
Rivers have taught fishermen a hard lesson: even small-scale chemical leaks leave a mark. Many metal carboxylates, including zinc isooctanoate, don’t break down easily in natural waters. Once they reach soil or waterways, microorganisms tend to struggle with them, and there’s the real risk of bioaccumulation in aquatic life. That’s worrisome. Elevated zinc in riverbeds, even from seemingly minor releases, tampers with growth, reproduction, and survival rates of fish and amphibians.
Some industry data points to a moderate environmental hazard, mainly in higher concentrations. The chemical isn’t as notorious as lead or mercury, but it still doesn’t vanish without a trace. Environmental watchdogs have started to track zinc isooctanoate in runoff and wastewater streams, especially when local fish populations suddenly dip or algae blooms overtake stagnant ponds. Spotting the source early, before wildlife numbers crash, is key.
When safety training gets brushed aside for the sake of speed or profit, that’s usually when accidents happen. Straightforward fixes go a long way: keep protective gear standard in plants, invest in updated filtration systems, and push for regular air quality checks. Product safety data sheets need a clear highlight on chronic hazards, not just immediate risks. I've seen management teams listen closely when staff bring up practical solutions, especially after a close call or minor scare.
Wastewater from manufacturing deserves attention, too. Encouraging operational audits and modern separator technology helps keep trace chemicals out of rivers. Strengthening partnerships between chemical companies and local environmental agencies tends to spot problems faster than audits alone. Community monitoring and hard science can work together to keep both workers and the ecosystem safer.
The story behind zinc isooctanoate echoes a bigger truth about specialty chemicals. Understanding, not just compliance, brings safer factories and cleaner rivers. Transparent reporting and responsible production ensure risks remain manageable and allow both health and environment to catch a fairer break.
| Names | |
| Preferred IUPAC name | zinc 3,5,5-trimethylhexanoate |
| Other names |
Zinc 2-ethylhexanoate Zinc octoate Bis(2-ethylhexanoato)zinc Zinc isooctanoic acid salt |
| Pronunciation | /ˈzɪŋk aɪsoʊˈɑktəˌnoʊ.eɪt/ |
| Identifiers | |
| CAS Number | 136-53-8 |
| 3D model (JSmol) | 3D model (JSmol) string for Zinc Isooctanoate: `C(C)(CCCC)CC(=O)[O-].C(C)(CCCC)CC(=O)[O-].[Zn+2]` |
| Beilstein Reference | 1776290 |
| ChEBI | CHEBI:91325 |
| ChEMBL | CHEMBL514330 |
| ChemSpider | 26737275 |
| DrugBank | DB11120 |
| ECHA InfoCard | 19b041c7-0923-47bc-991a-8b60471a3a85 |
| EC Number | 272-028-8 |
| Gmelin Reference | 23876 |
| KEGG | C15901 |
| MeSH | D001 Zinc Compounds |
| PubChem CID | 14822 |
| RTECS number | ZG8770000 |
| UNII | 5AIV4R8DWV |
| UN number | UN3082 |
| Properties | |
| Chemical formula | C16H30O4Zn |
| Molar mass | 427.1 g/mol |
| Appearance | Clear, colorless to pale yellow liquid |
| Odor | Odorless |
| Density | 1.1 g/cm³ |
| Solubility in water | Insoluble |
| log P | 3.83 |
| Vapor pressure | Negligible |
| Acidity (pKa) | pKa ≈ 4.9 |
| Basicity (pKb) | 6.35 |
| Magnetic susceptibility (χ) | Diamagnetic |
| Refractive index (nD) | 1.493 |
| Viscosity | 40 mPa.s (25°C) |
| Dipole moment | 2.58 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 668.6 J/(mol·K) |
| Std enthalpy of formation (ΔfH⦵298) | -531.42 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -4878.8 kJ/mol |
| Pharmacology | |
| ATC code | A16AX14 |
| Hazards | |
| Main hazards | May cause respiratory irritation. Causes serious eye irritation. May cause an allergic skin reaction. |
| GHS labelling | GHS07, GHS09 |
| Pictograms | GHS07, GHS09 |
| Signal word | Warning |
| Hazard statements | H317: May cause an allergic skin reaction. |
| Precautionary statements | IF ON SKIN: Wash with plenty of soap and water. If skin irritation occurs: Get medical advice/attention. Take off contaminated clothing and wash it before reuse. |
| NFPA 704 (fire diamond) | 1-1-0 |
| Flash point | > 230°C |
| Lethal dose or concentration | LD50 (Oral, Rat): > 5000 mg/kg |
| LD50 (median dose) | LD50 (median dose): >5000 mg/kg (rat, oral) |
| PEL (Permissible) | PEL: 5 mg/m3 |
| REL (Recommended) | 2500 mg/kg bw/day |
| Related compounds | |
| Related compounds |
Zinc stearate Zinc laurate Zinc palmitate Zinc myristate Zinc oleate |