
The journey of yttrium isooctanoate began with the wider commercial discovery and isolation of rare earth elements in the late nineteenth century. Unlike the flashier elements grabbing headlines in those early days, yttrium slowly carved a niche for itself in research labs and industrial processes through the twentieth century. Once industries started hunting for organometallic compounds that offered both oily stability and reliable reactivity, people found isooctanoates — with their unique carbon tails — promising partners for a host of metals. Mixing yttrium’s unusual electron configuration and moderate abundance with the grease-like properties of isooctanoic acid opened the door to a compound that merges chemical purpose with physical convenience. Early use cases popped up across niche research, but as rare earth chemistry matured and specialty catalysts became valuable, yttrium isooctanoate showed up more regularly on technical datasheets and chemical order forms.
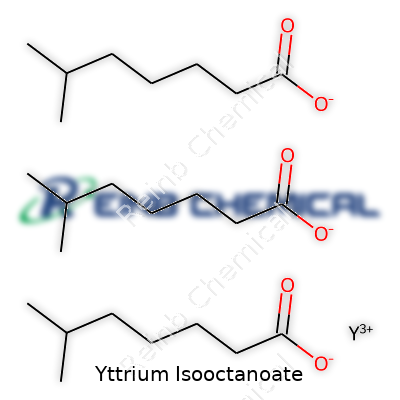
Yttrium isooctanoate sits in the family of carboxylate metal salts, offering a way to handle yttrium outside the brittle world of oxides and chlorides. The compound combines the central yttrium ion with isooctanoate anions, which brings a degree of oil-like solubility and organophilic behavior. Its formula often reads as C24H45O6Y, and its structure allows it to dissolve in most organic solvents while resisting easy hydrolysis. This form makes it appealing in both reaction chemistry and as a raw material for coatings, electronics, and even research-grade lubricants. On the shelf, it usually appears as a colorless to pale yellow liquid or viscous oil, depending on trace impurities and synthetic routes.
Chemists who handle yttrium isooctanoate notice its oily consistency right away. The substance doesn’t handle water well, showing only sparing solubility in aqueous phases; drop it into benzene, toluene, or xylene, and it goes in smoothly. Its density ranges between 1.02 and 1.08 g/cm3 at room temperature, with a light breakout of characteristic organic odor. Melting points vary, but one expects a pourable substance even below standard lab room temperatures. Chemically, yttrium holds a +3 oxidation state here, tucked inside the shield of three bulky isooctanoate anions. The compound stays largely stable in storage, provided containers avoid strong acids or bases, humidity, and reactive oxidizers. Thermal stability keeps up into the 200°C region before decomposition bites, making it fit in moderate-temperature syntheses.
On packaging, yttrium isooctanoate often sports a purity label swinging from 98% to 99.9%, driven by the intended market. Impurity analysis focuses on iron, rare earth contaminants like cerium and lanthanum, chloride residues, and moisture content. Manufacturers provide technical sheets listing everything from molecular weight (~537 g/mol) to solvent compatibility, and label containers with the CAS number (often 33953-13-2), hazard designations, and storage guidelines including dark, ventilated conditions. It usually ships in sealed metal or high-density polyethylene vessels, with clear hazard icons for eye and skin contact. Barcodes, batch tracking, and country-of-origin stamps round out standard modern labeling requirements.
Synthesis starts with a high-purity yttrium oxide or chloride as the precursor. Chemists dissolve the yttrium salt in an organic solvent like toluene, then add isooctanoic acid under slow agitation. Reaction proceeds by sluggish yet steady metathesis, where yttrium swaps its native anions for three isooctanoate arms. Sometimes the mix receives a dash of dehydrating agent to strip away water, driving the chemical equilibrium toward complete conversion. On cooling, the reaction mass transitions to a slick, low-viscosity liquid. Filtration and solvent evaporation finish the process. People who care about purity usually push one or two extra washes with dry organic solvent, then dry carefully under reduced pressure. The final compound has to meet impurity, moisture, and stability benchmarks before release for sale or lab use.
Yttrium isooctanoate shows surprising flexibility in organic synthesis. Tossing it into a batch reaction delivers yttrium in an organophilic, mostly non-ionic form, allowing controlled metalation or serving as a precursor to yttrium-based catalysts. Heating in air or in the presence of strong oxidizers converts it to yttrium oxide, handy for producing fine particulate films or thin-layer ceramics. Chemists sometimes tweak the isooctanoate tails to build mixed carboxylate species, tuning both solubility and reactivity for special applications. In some modern research, this reactivity feeds into cross-coupling reactions or as an additive in polymerization runs, especially where gentler metal donors outperform old-school salts.
Chemical catalogues and patents lean on a handful of accepted synonyms. One runs into “yttrium(III) isooctanoate,” “yttrium isooctanoate carboxylate,” or “yttrium caprylate” in trade lists. Brand-name versions rarely stray far, often appending the manufacturer’s logo, purity percentage, or an in-house reference code. Internationally, labels may switch between “isooctanoic acid yttrium salt” or its more formal IUPAC variant. Digital product listings highlight these names for procurement searches and customs declarations.
Yttrium compounds historically raise eyebrows over inhalation or chronic exposure risk. Isooctanoate esters moderate some of the bioavailability concerns, but gloves and goggles remain table stakes in the lab. The oily base can slick up glass and metal surfaces. Fume hood procedures catch any vapors or accidental splashes. Absorption through skin sits on the low side among rare earth derivatives, but eye contact causes irritation and persistent dryness. Technical safety data sheets outline emergency steps for spills, accidental ingestion, or combustion (the latter producing acrid, asphyxiant fumes). Chemical handling regulations extend to mid-scale industrial settings, where vapor extraction and liquid containment prevent environmental loading. Most modern laws avoid strict toxicity labeling but require material safety data retention and restricted access in campus and commercial settings.
Anyone working in specialty catalysis, polymer modification, or surface coatings will see yttrium isooctanoate at some point. Its soluble, oil-friendly format fits neatly into formulations that need a gentle metal donor without the grit and speckling of metal powders or simple salts. In paints and drying agents, it promotes effective polymer cross-linking at room and low-bake temperatures, cutting down reaction times for industrial lines. Electronic engineers eye it for thin film deposition, where yttrium’s magnetic and dielectric properties bring material advantages for capacitor dielectrics and high-performance insulators. Sometimes, lubricants and high-temperature greases grab it for metal reinforcement. Research settings, from academic chemistry to advanced materials centers, run through it in the search for novel metal-organic frameworks or as dopants in luminescent glass and ceramics.
Lab groups hoping for next-generation functional materials often pick yttrium isooctanoate for its clean conversion to yttrium oxide in thin-film applications and for its predictable metal delivery in solution-phase synthesis. There is always a drive for higher-purity, lower-residual-solvent grades, with nanotech and green chemistry circles pushing greener, less hazardous process variants. As new organic ligands roll out for designer applications, chemists benchmark yttrium isooctanoate for solubility, thermal stability, and reactivity adaptation. Emerging research explores coupling with phosphors for lighting, doping in optical fibers, and as a base for quantum-dot precursors. The combination of moderate cost, flexible application, and regulatory neutrality gives it staying power in academic and commercial research portfolios.
Toxicologists keep tabs on yttrium compounds, and studies show the isooctanoate ester shows low acute toxicity on skin and through inhalation at typical lab concentrations. Chronic exposure over months or years — as with most rare earths — can build up in bones or soft organs, raising flags for occupational safety but not banning its use outright. Animal models reveal slow excretion, with most effects linked to massive, repeated exposures. Remembering some rare earths produce subtle neurological tweaks at high levels, industrial standards press for routine glove and mask use. Waste disposal follows hazardous-organic-waste guidelines, not general trash. Outreach between chemists and medical researchers continues, searching for clearer markers on long-term exposure and better guidance on safe limits, especially in manufacturing.
With industries craving smarter materials and more efficient electronic films, yttrium isooctanoate will likely grow in profile over the next decade. Its easy handling makes it a candidate for scaling up yttrium-based ceramics and energy-efficient coatings. Developments in rapid additive manufacturing and materials-by-design approaches depend on reliable, well-characterized chelated rare earths, and this compound delivers on both counts. As electric vehicles and alternative energy ramp up demand for rare earths, the push for recycling and greener synthesis methods could refine current production methods further. Research into lower-toxicity analogs, tighter regulatory control, and broader application mapping continues among both industrial and university labs, with global trends shaping supply, demand, and innovation pace.
Yttrium Isooctanoate may sound like a mouthful you’d only hear tossed around in a high-tech lab, but its impact stretches far from the test tube. This chemical compound brings yttrium, a rare earth element, together with isooctanoate – a component commonly found in metal soaps. The result is a specialty material that has crept into several industries, shaping outcomes in subtle but meaningful ways.
Painters and manufacturers have used metal soaps for decades. Slide yttrium into the mix, and you get one with distinct properties: yttrium isooctanoate serves as a catalyst, helping chemical reactions along without breaking down itself. This comes in handy. Many companies use it as a drying agent in the coatings industry. Coatings with metal soaps dry more evenly, resist scratches better, and stand up to weather with less fuss. Oils used in paints, varnishes, and inks often need partners like yttrium isooctanoate to ensure they cure and protect what they cover.
Over in the world of plastics, yttrium isooctanoate steps in to give thermal stability. Polymers subjected to high temperatures can break down, losing shape and function. Introducing yttrium keeps the entire process running smoother. From appliance housings to auto parts, durability often comes from little tweaks like this at the chemical level.
For folks working in manufacturing, reliable catalysts and stabilizers make a difference. Time means money, and curing time can make or break a production schedule. A drying agent or plastic stabilizer that falls flat could mean sticky floors, warped goods, or an extra bill for wasted materials. With yttrium isooctanoate, producers nudge their products closer to repeatable, dependable quality. I remember seeing a batch of paint jobs ruined by unpredictable drying—one change in the formula fixed it and that fix included a catalyst just like this one.
Rare earths come with concerns. Mining and processing yttrium, though not as invasive as some heavy metals, drag along questions of sourcing, labor rights, and waste. More companies look for ways to recycle chemicals in closed-loop systems or source specialty chemicals from traceable operations. In the EU and US, strict regulations push for safer, greener approaches. The world continues to invent smarter solutions for disposing or reusing chemical residues without hefty waste piles.
Anyone buying specialty chemicals faces supply chain headaches and price jumps. Global uncertainty—for example, shutdowns in major mining regions—ripple through the market. One way around this problem is to recycle and reuse waste catalysts in manufacturing. Research keeps opening up new paths: scientists work on alternate drying agents that don’t rely on rare earth elements, giving industries more options and price stability. It reminds me of the old lead-based paint pigments that faced a wave of innovation and regulation until safer, high-performing substitutes took over.
Demand for efficient, high-performing materials never rests. From what I’ve seen, the real value of yttrium isooctanoate comes down to the details: helping products perform better, cutting back on defects, and unlocking new features in cherished stuff like paints and plastics. With the right attention to sourcing and environmental care, these specialty chemicals offer a glimpse into the complex, sometimes hidden ways science shapes daily life.
Yttrium is a less-famous rare earth metal that turns up in a surprising number of technologies. It might not catch headlines like lithium or neodymium, but its compounds often show up in electronics, ceramics, and catalysts. Isooctanoate, sometimes known as 2-ethylhexanoate, steps in as a common organic ligand in metal-organic chemistry. Pair these two together and you get yttrium isooctanoate—a chemical that brings together the unusual strengths of each component.
Yttrium isooctanoate’s formula starts with yttrium’s usual trivalent state. Yttrium ions typically take the +3 charge, which matches up with three isooctanoate anions—each carrying a -1. The chemical formula ends up as Y(C8H15O2)3. That string might look like a mouthful, but it tells the story. Isooctanoate brings a long carbon chain, giving the compound an organic character that helps it dissolve into non-polar solvents, unlike simple yttrium salts.
The C8H15O2 part stands for the isooctanoate ion, which results from removing a proton from isooctanoic acid (2-ethylhexanoic acid). String three of those ions onto a yttrium center, and the charge balances out.
A clear chemical formula isn’t just trivia. A heavy element like yttrium, when paired with an organic ligand, shapes the way the whole material behaves. The formula guides how labs synthesize the compound, predicts how it might dissolve, and hints at its stability. You can’t even think about quality control or reproducible research without confidence in that basic knowledge.
I used to do metal-organic syntheses in grad school. Getting a formula wrong could set you back days, if not longer. Wrong stoichiometry meant mystery solids, or oils where crystals were promised, or worse—thinking you had the right product when you didn’t. With a robust formula like Y(C8H15O2)3, researchers track purity by comparing elemental analysis or NMR results to the numbers predicted straight from that equation.
Yttrium isooctanoate isn’t a blockbuster compound for consumers, but it’s a quiet workhorse in specialty chemical processes. Its ability to dissolve in organic mixtures makes it valuable for coatings, fuel additives, and even catalysts in polymerization. Manufacturers use yttrium isooctanoate for surface modification in electronics and optoelectronic films because the organic tails play nice with different materials.
Increasing demand for rare earth elements pressures both miners and chemists to account for every gram. Formulation mistakes could threaten the bottom line or yield unwanted waste. Keeping formulas public and trustworthy helps avoid counterfeits or misrepresented batches—a real risk in today’s global chemical market, especially with rising economic stakes around rare earths.
Strong documentation and open data matter. I saw how a little ambiguity in a chemical’s formula could turn a promising process into a dud. Real consequences came from small errors. Ensuring clarity—like nailing down Y(C8H15O2)3 as yttrium isooctanoate—means smoother operations, less wasted effort, and safer lab practices. Double-check the sources, consult reputable suppliers, and use analytical tools to support every claim.
A clear question comes up any time new materials land on a workbench: How safe is it to handle? Not everything with a scientific name is dangerous, but it sure asks for respect. Yttrium isooctanoate lands in that zone where you don’t see a dozen warning signs, but you also won’t catch seasoned techs treating it like free candy.
Anyone handling metal carboxylates, including yttrium isooctanoate, learns early that a simple pair of gloves grants a surprising amount of confidence. This stuff isn’t as temperamental as classic lab hazards like sodium or mercury, so you won’t see anybody sweating when a new container gets opened. Yet, the phrase “better safe than sorry” has real weight in a research setting.
Contact with yttrium isooctanoate probably won’t burn holes through your gloves, or fog up the fume hood. Safety sheets point to low acute toxicity, but they also mention skin and eye irritation—things that stack up after repeated use if folks get lazy about basic safety gear. Even seasoned techs have a story about that time they skipped goggles for “just a minute,” earning a red face and a quick lesson in humility.
Plenty of safety data stops at “may irritate” for compounds like this. Acute toxicity in animal studies shows high tolerance, putting it on the lower end when ranked with industrial chemicals. It’s not blowing up in air or leaching poison through skin, yet inhaling dust does rough up lungs. That dust proves tricky for any powdered or granulated material, not just yttrium compounds.
Repeated exposure draws more attention than a one-off spill. Chronic handling—especially without gloves or masks—could inflame skin or respiratory systems, leading to those familiar rashes or coughs anyone in materials science can recognize. Proper labeling is more than a legal demand; it becomes the difference between a clean shift and an unplanned doctor visit.
Even “safe enough” chemicals have a habit of catching out overconfident users. It’s easy to grow casual, skipping masks or proper clean-up, especially during a long day. Yttrium isooctanoate won’t melt skin on contact, but complacency grows risks over time. Fume hoods, safety goggles, and gloves create an easy routine for a reason. Folks often forget: dust, spills, or accidental mixing never sends a warning email before trouble starts.
Many labs deal with dozens of similar compounds. The labs that avoid problems keep two things in mind—respect for every container, and a backup plan for accidents. Fast access to eyewash stations, running water, and spill kits lets even messy situations get handled without drama. Supervisors who walk their talk—demonstrating safe handling, not just talking about it—set the real culture for a safe lab.
Sheet after sheet of guidance point to three basics: cover your hands, shield your face, and tidy up any spills right away. Even the least hazardous compounds slowly pile risks over years, especially with lax habits. Regulations also shift; what’s permitted today might earn a fine tomorrow.
For the experienced tech, yttrium isooctanoate looks manageable. For the newbie in an academic lab, clear rules, signage, and sharp reminders from mentors make a bigger difference than any official warning. Training refreshers—boring as they seem—keep mistakes rare. This approach stretches from research labs to factories: respect the chemical, respect your colleagues, and the workday wraps up without surprise trouble.
Yttrium Isooctanoate leans toward specialty applications, especially in plastics, paints, and some high-performance glass production. People value it for its ability to modify surfaces and improve physical properties. Handling and storing this chemical shouldn’t feel like guesswork, considering people’s health and environmental safety ride on these choices. I’ve watched labs—big and small—deal with metal-organic compounds and witnessed how careless storage leads to headaches down the line. Cracked containers, ruined batches, or worse, safety scares. Cycles of stress most workers never sign up for, all because proper storage takes a back seat to convenience.
Yttrium Isooctanoate usually arrives in a liquid form, often pale or clear, and it gives off a distinct odor. It’s not one you forget quickly after spending long days in a lab. Left exposed or at the wrong temperature, this compound breaks down or gets contaminated. Moisture is the hidden enemy here. Even a little humidity creeping through a container's lid starts a chain reaction, creating unwanted byproducts or weakening the compound’s effectiveness. Beyond performance, a leaky drum or broken bottle means risk to workers—direct skin contact brings irritation fast. Respiratory issues show up if vapors hang in an unventilated storeroom. These problems aren’t distant threats. Companies pay for slip-ups with wasted product, medical bills, or even run-ins with the environmental authorities.
I’ve seen good storage standards keep doors open and crews healthy. Every time, it comes down to three things: isolation, climate, and labeling. Store Yttrium Isooctanoate in tightly sealed drums or bottles made of glass or a tested polymer. The seals have to work, not just sit in there for show. Warehouse teams should check caps and gaskets at every delivery and before anything goes back on the shelf. Choosing a cool, dry chemical storeroom prevents temperature swings from turning this compound unstable. Think less than 25°C and humidity under 60%. Most trouble starts when chemicals end up near water lines or open windows. Even a slow drip can spell disaster after a few days.
Every label should show the correct name, hazard icons, and the date received. No scribbles, no makeshift stickers. If someone has to squint to read a date or guess what’s inside, that’s a problem waiting to happen. It pays off to store Yttrium Isooctanoate separate from acids and bases—reactivity is always a risk. I’ve watched near-misses get averted because a junior tech spotted a misplaced bottle and moved it before the night shift arrived. Training staff to look for leaks, check labels, and update logs cuts down on mistakes fast. Salting a few regular safety audits into the routine shouldn’t feel like a burden. It’s a reality check for the whole team.
Personal experience taught me never to put off minor fixes in a storeroom. Leaky seals, blocked ventilation, or unlabeled bottles become tomorrow’s disaster. Respirators, gloves, and splash-proof goggles are not optional, even for a splash that seems minor. Anyone working with Yttrium Isooctanoate earns better protection than penny-pinching owners or lazy supervisors want to give. Documenting any changes in storage routine and encouraging open conversation around spills or exposures will go further than any laminated poster. Prioritizing responsible care means cleaner workspaces and fewer near misses—something everyone wants, but only happens when the basics get real attention, every single day.
People in chemistry and manufacturing circles often get into debates about purity levels, especially when the material in question ends up driving performance in advanced tech. Yttrium Isooctanoate is one of those compounds where purity isn’t just a number—it changes how projects turn out and how much trust partners can put in a supplier’s word. Walk into a specialty lab or a coatings manufacturing plant and mention purity below 98%, and you’ll probably see a few raised eyebrows. Many researchers and engineers stick with material in the 98–99.5% range, and a growing number are only happy with 99.9% grades for projects where failure carries a big price tag.
Purity sounds like a salesperson’s pitch, yet it ends up playing a huge role in things that most people never see. Think about electronics: low purity can introduce trace metals that scramble signals or even threaten battery lifespan when used in energy storage applications. I’ve worked in labs where a single batch of slightly contaminated Yttrium product slowed everyone down for days, since extra time had to go into additional purification rather than progress.
Errors in purity make ripples throughout a workflow. For example, in OLED manufacturing, unexpected trace metals from a 95% pure supply caused color shifts and instability. This is real money and lost opportunity, not just paperwork. The purity level isn’t just a statistic, it’s a guardrail for reliability. If a supplier advertises “high purity” and labs still spot 0.5% unknowns, that product is often pushed to the back of the shelf, only used when absolutely necessary or never again.
Manufacturers use a mix of chemical titration, spectroscopy, and sometimes even chromatography to check what’s in a drum of Yttrium Isooctanoate. In my own lab days, a certificate of analysis meant we could see breakdowns of impurity levels, sometimes down to the parts per million. The companies earning steady business show this documentation upfront, often sharing the exact test method they use, not just a summary. Without transparency, many scientists and engineers feel forced to treat every new batch as a wildcard, spending valuable hours confirming what should already be certain.
Demand has never been higher among manufacturers working on magnets, displays, and catalysts. Every year, applications get more demanding and the number of suppliers promising “ultra-high purity” grows, but not all live up to the promise. Stories of surprise contaminant findings are still too common, especially for traders who buy from middlemen instead of direct from trusted producers. I’ve seen startup teams excited about a new project get stalled by one batch of Yttrium Isooctanoate testing below the promised threshold, cutting months off of development time.
Higher standards don’t just happen in isolation. I’ve watched scientific collaboration prompt suppliers to improve their processes. Sharing test results and actually listening to feedback helps weed out unreliable batches faster. Certification programs and regular audits keep suppliers honest and motivated to keep purity high. Instead of waiting for problems, teams encourage proactive transparency, choosing partners who show they care about rigorous standards. By talking openly and holding suppliers accountable, the bar for purity rises in real, measurable ways.
| Names | |
| Preferred IUPAC name | 2-Ethylhexanoic acid; yttrium(3+) salt (3:1) |
| Other names |
Yttrium(III) 2-ethylhexanoate Yttrium 2-ethylhexanoate Yttrium octanoate Isooctanoic acid yttrium salt |
| Pronunciation | /ˈɪtri.əm aɪˌsəʊˈɒk.tə.neɪt/ |
| Identifiers | |
| CAS Number | ["13494-98-9"] |
| Beilstein Reference | 3853080 |
| ChEBI | CHEBI:53253 |
| ChEMBL | CHEMBL514417 |
| ChemSpider | 17340545 |
| DrugBank | DB14538 |
| ECHA InfoCard | 200-582-1 |
| EC Number | 291-235-5 |
| Gmelin Reference | 1315700 |
| KEGG | C18688 |
| MeSH | D015440 |
| PubChem CID | 163309247 |
| RTECS number | UJ8750000 |
| UNII | V3O95F285Y |
| UN number | UN3334 |
| CompTox Dashboard (EPA) | DTXSID7041573 |
| Properties | |
| Chemical formula | C16H31O4Y |
| Molar mass | 676.379 g/mol |
| Appearance | Clear pale yellowish liquid |
| Odor | Odorless |
| Density | Density: 1.12 g/cm³ |
| Solubility in water | Insoluble in water |
| log P | 2.44 |
| Vapor pressure | Vapor pressure: negligible |
| Basicity (pKb) | pKb ≈ 5.92 |
| Refractive index (nD) | 1.490 |
| Viscosity | Viscosity: 160 cP |
| Dipole moment | 1.72 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 713.6 J·mol⁻¹·K⁻¹ |
| Pharmacology | |
| ATC code | V10BX03 |
| Hazards | |
| GHS labelling | GHS02, GHS07 |
| Pictograms | GHS05,GHS07 |
| Signal word | Warning |
| Hazard statements | H315: Causes skin irritation. H319: Causes serious eye irritation. H335: May cause respiratory irritation. |
| Precautionary statements | P264, P280, P305+P351+P338, P337+P313 |
| Flash point | > 113°C |
| Lethal dose or concentration | LD50 (oral, rat): >5000 mg/kg |
| LD50 (median dose) | LD50 (median dose): >5000 mg/kg (rat, oral) |
| NIOSH | Not established |
| PEL (Permissible) | PEL (Permissible Exposure Limit) for Yttrium Isooctanoate: 1 mg/m3 (as Yttrium) |
| REL (Recommended) | 10 mg/mL |
| Related compounds | |
| Related compounds |
Yttrium(III) oxide Yttrium(III) acetate Yttrium(III) chloride Yttrium(III) nitrate |