
Vanadium’s story stretches back more than two centuries, but vanadium isooctanoate represents a much newer chapter. In the 20th century, chemical researchers searching for ever more efficient catalysts drew from the early industrial use of vanadium pentoxide. By the 1980s, engineers had experimented with various vanadium carboxylates as paint dryers and oil additives, tinkering to improve solubility in organic systems. Vanadium isooctanoate came about as a practical solution, drawing on metal-organic synthesis techniques that saw broad expansion thanks to improvements in organic chemistry. Many companies worked on proprietary production methods to answer the growing demand from lubricants and fuel additive industries. Patents published in the 1990s detail specific improvements, such as the use of tailored ligands or stepwise purification, actively shaping how the market for this compound took shape.
Vanadium isooctanoate sits as a specialized metal-organic compound, best known for its role in catalysis and chemical process industries. Its structure, vanadium bonded to fatty acid chains derived from isooctanoic acid, gives it desirable oil-soluble characteristics, which chemists in industrial labs usually look for when targeting non-polar applications. Companies typically ship it as a deep orange to red liquid, packaged tightly to prevent exposure. This compound often finds use as a drier in paints and varnishes, and also works as a chemical intermediate in fuel formulations and polymer reactions. Its relative stability in liquid state allows storage and transport under common industrial conditions, though certain handling considerations remain.
In daily life at a chemical plant, vanadium isooctanoate sits as an oily, colored liquid with a pungent odor. Its molecular weight depends on the degree of polymerization, but commercial grades often report around 450-520 g/mol. It dissolves well in non-polar solvents like mineral oils and aromatic hydrocarbons, but stays away from water. Typical density floats between 0.9 and 1.1 g/cm³, and its viscosity stays relatively high, making pouring and mixing a slow affair without heating. The compound starts breaking down at increased temperatures above 150°C, with metal-organic bonds snapping and releasing both vanadium oxides and volatile acids. On the chemical side, the vanadium atom sits in an oxidation state between +3 and +5, giving the compound redox potential that proves useful in specific chemical processes.
Anybody handling vanadium isooctanoate in an industrial setting pays close attention to its technical sheets. Producers commonly specify vanadium content by percentage, often targeting 8 to 12% by weight for standard grades. Labels require details about solvent ratio, trace impurities like sulfur or chlorides (which could interfere with catalysis), and any stabilizers added to prevent premature oxidation. Governments and industry guidelines mandate hazard pictograms showing toxicity and environmental risk. Documentation includes batch numbers, production dates, and expiration timelines, since exposure to air or moisture can lower quality. Manufacturers frequently follow ISO and REACH regulations, not just for safety, but to ensure consistency across batches that end up in paints, oils, or chemical reactors worldwide.
Making vanadium isooctanoate involves a fairly direct reaction between vanadium pentoxide or vanadium oxytrichloride and isooctanoic acid in an organic solvent like toluene or xylene. Technicians charge reactors under an inert atmosphere, because vanadium compounds react vigorously with air and moisture. They gradually add isooctanoic acid under controlled temperature, monitoring both the acid number and vanadium content at set intervals. When the reaction reaches target conversion, operators remove byproducts such as water or hydrochloric acid by distillation. Modern setups rely on vacuum systems and moisture scrubbing to keep the product pure and avoid corrosion of plant equipment. Using tight temperature and time controls coaxes maximum yield from each batch, while impurities get removed through filtration or solvent washes before packaging.
Vanadium isooctanoate’s redox power makes it stand out in chemical processes. In catalysis, operators use its vanadium center to shuttle electrons during oxidation reactions, breaking down large hydrocarbon molecules in oil refining. Blending with other metal carboxylates allows fine-tuning of drying time in paints at an industrial scale. It also enters into transesterification reactions, forming part of alkyd resins and polyesters. Chemists can modify the isooctanoate ligands, swapping in other branched or straight-chain acids to better suit either solubility or reactivity needs. Exposure to air or strong acid will eventually break down the compound, so storage calls for dry, airtight conditions and limited shelf life if opened. Engineers designing new catalysts continue to explore its structure, searching for balance between vanadium's redox properties and overall molecular stability.
In lab and trade catalogs, vanadium isooctanoate also appears as vanadium(III) isooctanoate, vanadium neodecanoate, or “VOOT” (Vanadium Octoate). Industry shorthand often skips to "vanadium drier" in paint and coatings contexts, though this umbrella term includes other carboxylates too. Suppliers may list custom blends under proprietary names, but technical data sheets reveal the key active as vanadium isooctanoate or its close structural cousins. Users double-check CAS numbers and molecular formulas to avoid confusion, since some vendors swap ligands but still advertise under similar branding. Cross-checking ensures the compound’s stated percentage matches technical needs in the field, preventing costly mix-ups in formulation or regulatory compliance.
People working with vanadium isooctanoate respect its safety demands. Spills leave oily stains that resist water cleanup, and direct skin contact can irritate or cause sensitization over repeated exposures. Industrial hygiene involves gloves, goggles, and, in some cases, respirators to guard against vapor inhalation. Facilities follow strict storage: cool, dry spaces with proper secondary containment, away from acid or oxidizing agents that could trigger unwanted reactions. Employees keep fire extinguishers nearby, since both the compound and its solvents pose fire risks. Waste disposal sees extra attention, with spent material collected for chemical incineration or specialized hazardous-waste landfilling to avoid groundwater contamination. Training forms the backbone of prevention, and regular audits help keep staff prepared in case of leaks or spills. Regulatory bodies in the US, Europe, and Asia set clear guidelines for permissible exposure, shipping, and emergency action, with non-compliance risking not just fines but harm to worker health.
Vanadium isooctanoate shows up mainly in drying agents for alkyd paints and varnishes, where it speeds up film formation for improved touch-dry times in construction and manufacturing. Fuel additive companies use it to enhance the performance of marine and heavy fuel oils, improving combustion and lowering soot or residue in diesel engines. Some polymer chemists tap it as a catalyst during polyester or polyamide manufacture, seeking selective reaction without introducing water-soluble contaminants. Laboratory researchers also use it in small-scale synthesis where redox-active metal complexes offer a clear mechanistic advantage. New applications drift toward lithium-ion battery production, especially as researchers seek high-voltage cathode materials that rely on transition metals’ unique electron-shell structures. These new routes rest on established safety protocols, since reactivity outside controlled systems risks unexpected hazard.
Research groups focus on vanadium isooctanoate for both process optimization and new-field exploration. Scientists examine ligand modification, shifting from isooctanoic acid to longer or more branched chains to improve dispersion in emerging polymer systems. Analytical chemists look into new spectroscopic methods (like NMR and FTIR) to track degradation during prolonged storage or intense medicinal application. Industrial-scale producers test green chemistry approaches, often trying to swap out toxic or high-cost solvents for bio-based or recyclable media. Students and postdocs research its redox action in catalysis, measuring electron transfer rates and comparing them to older lead-based or cobalt-based driers, aiming to move coatings and paint lines away from heavy metals. Regulatory researchers also analyze its breakdown pathways in the environment, tracing what happens after paint dries or lubricants burn, since the fate of vanadium compounds in soil or water influences new restrictions and permissible-use trends.
Vanadium isooctanoate’s health profile attracts close scrutiny. Traditional toxicology work shows that inhaling vanadium compounds over time can damage lungs or cause bronchial inflammation. Lab animal studies link high doses to kidney strain and possible neurotoxicity, though real-world exposures rarely reach such levels with modern workplace controls. Chronic skin contact can lead to dermatitis in sensitive individuals, especially where personal protective equipment lacks enforcement. Some vanadium carboxylates pass through biological membranes, raising concerns about reproductive toxicity and environmental persistence, since they do not easily degrade in soil or water. Regulatory agencies urge routine monitoring of vanadium levels in at-risk workplaces, and encourage medical checkups for employees in production or handling roles. Recent research seeks safer alternatives and tighter, lower-threshold exposure limits, guided by improved detection methods that catch vanadium PPB levels in human tissue or environmental samples.
Looking ahead, vanadium isooctanoate stands on shifting ground. Changing environmental standards push industries to lower heavy-metal content in paints and fuels, raising the bar for safe, effective alternatives. At the same time, battery researchers study transition metal carboxylates for energy storage, betting on vanadium’s stable redox cycles to anchor next-generation lithium or sodium cells. Some companies invest in greener production routes, aiming to capture spent vanadium from paints or industrial waste, recycling it back into fresh batches with smaller carbon footprints. Regulatory bodies plan to introduce updated Occupational Exposure Limits and require even stricter reporting on chemical releases. For vanadium isooctanoate, progress comes from blending long-learned chemical know-how with real-world practicalities, ensuring new uses grow in step with health and environmental stewardship, not just technical advances.
Industry folks have seen certain metals make big changes in how things get built, and vanadium’s impact in steel shows up everywhere—from the backbone of bridges to the nuts and bolts in your car. Vanadium Isooctanoate, a liquid compound, gives manufacturers more control during steel production. While working in a fabrication shop, I saw firsthand how quality hinges on even the tiniest chemical tweaks, and vanadium-based additives made weld joints stand up to years of rough use. Engineers often prefer vanadium additives for their knack at increasing strength without cranking up the cost. Corrosion slows down, toughness ramps up, and project budgets breathe a sigh of relief.
Vanadium Isooctanoate steps up as a catalyst in oil refineries. Breaking down heavy crude into something useful, like gasoline or jet fuel, demands serious efficiency. Catalysts set the pace. Having worked with refinery teams, I know any boost to run times or reduction in gunk build-up matters. This compound helps refineries squeeze more product out of the same barrel, which means less waste and more value for every shipment. Prices at the pump feel the ripple effect. No fancy tricks or empty promises—just a chemical that quietly sharpens the bottom line for refiners.
Vanadium Isooctanoate doesn’t only stick with metals and fuels. Paint and coating manufacturers keep returning to it as a drying agent. In the shop, we needed coatings to cure fast and set hard, especially during unpredictable weather. This compound helps shorten production times and minimizes headaches with tacky surfaces. Protective finishes, from marine vessels to farm tools, get a leg up on durability thanks to the subtle chemistry at work. You end up with fewer callbacks for peeling or rusted finishes, making everyone’s job easier.
Plenty of chemicals churn through industry, but not many escape a microscope. Vanadium compounds, Isooctanoate included, get a close look for environmental impact. Safety rules shape every step from delivery to disposal. In my own work, the focus shifted toward responsible sourcing and closed-loop handling. Cutting corners can ruin reputations fast. That’s why companies lean on traceable supply chains and regular lab checks. Independent audits and transparent data keep buyers and regulators confident that risks stay in check.
As batteries and renewable tech pick up steam, metals like vanadium attract more research. Vanadium Isooctanoate sits in a toolbox alongside other specialty chemicals, waiting for a call as new uses pop up. Reliability and value drive its adoption—key lessons learned in every factory floor meeting and jobsite briefing I’ve attended. If industries keep looking for performance and accountability, interest in this compound won’t fade anytime soon.
Vanadium compounds pop up more often in industry than most folks expect. Vanadium isooctanoate, for instance, pulls its weight in the world of catalysts. The chemical formula of this substance is VO(Octanoate)2, most commonly simplified as C16H30O4V. For those who spent high school chemistry dodging the details, this formula reflects vanadium metal paired with two isooctanoic acid residues. The isooctanoate part stems from a branched version of the trusty old caprylic acid molecule. Vanadium, parked at the center, acts as the backbone.
Getting this formula right goes way beyond textbook trivia. Raw numbers and atoms mean something real on the production line. Miss the balance and a batch of catalyst loses its punch. Vanadium isooctanoate often finds its home in industries pushing for high-octane fuel. The formula, carved in stone by chemistry rules, gives certainty over things like reactivity, solubility, and the way it plays with other materials. Someone forgets that vanadium stands at the +4 oxidation state here, and suddenly, the replacement behaves completely differently.
I’ve worked around metal-based catalysts before, and the difference in a formula ripples down to the smallest equipment setting. Imagine tossing in extra oxygen by confusing vanadyl (VO2+) with vanadate (VO43–). You don’t just waste money—you risk dangerous side reactions and even equipment fouling. This is one of those moments where a sharp eye for molecular symbols saves time and extends far beyond a classroom quiz.
Compounds with vanadium have a history with both opportunity and risk. On one hand, the formula C16H30O4V means the molecule holds enough organic matter to stay oil-soluble, making it a favorite for motor oil additives meant to fight engine wear or knock. On the other, people working anywhere near such chemical agents face strict exposure guidelines. Vanadium itself, in various forms, triggers respiratory irritation and chronic health problems if safety lines are crossed. Knowing that isooctanoate groups ride along affects how a spill gets cleaned up or contained, so that formula on a drum isn’t just a label—it’s a life preserver.
Information about substances like vanadium isooctanoate needs to come from reliable scientific studies and official databases. I’ve watched colleagues burn hours chasing rumors in outdated safety sheets. Seeking authoritative confirmation, such as the PubChem entry or a supplier’s certificate of analysis, steers clear of mistakes and ensures compliance with local regulations. Transparent sourcing and batch tracking hang on genuine, verified chemical formulas.
A formula like C16H30O4V unlocks conversations between researchers, plant engineers, doctors, and regulators. Clear formulas let teams talk about toxicity, storage conditions, and even transportation. Overlooking the detail invites confusion, lost time, and sometimes worse. For anyone who handles specialty chemicals, getting the formula checked and double-checked before use stands as a line between smooth operation and an expensive lesson.
Safer handling of metal-organic compounds calls for investing in regular training and updated labeling at every part of the chain. Chemical literacy—being able to interpret and apply these formulas—should be a shared priority. Digitized inventories using reputable databases can catch specification errors before purchases finalize. Giving operations folks a clear formula, together with specifics on health and fire risk, becomes not only smart, but necessary.
Whenever a novel compound turns up in a process, it pays to bring in both experienced chemists and those who know the nuts and bolts of the shop floor. Only a well-rounded, fact-based approach prevents trouble and saves real money. Vanadium isooctanoate’s formula sums up more than atoms—it shapes the practices around people and products in the real world.
Talk of vanadium compounds usually pops up in the context of heavy industry or catalysts in manufacturing. Vanadium isooctanoate shows up in paints, coatings, and sometimes in lubricant additives. Its job is to act as a chemical helper, making processes run smoother or products last longer. The stuff isn’t sitting on grocery store shelves, but it’s not out of the reach of workers in these industries.
Vanadium catches attention because certain forms—not all—can be toxic. Breathing in vanadium dust or fumes from heating compounds like isooctanoate can irritate the lungs. Studies of workers in smelting or alloy industries reported coughing, wheezing, and even bronchitis when dealing with vanadium pentoxide. It’s not the sort of exposure anyone shrugs off.
A Canadian Centre for Occupational Health and Safety summary highlights that vanadium compounds may irritate the eyes, skin, and airways. Larger parts of the world, from the U.S. to Europe, have safety guidelines for these chemicals. The American Conference of Governmental Industrial Hygienists recommends keeping average exposures far below what might bring on symptoms.
Vanadium compounds get into the body mainly through breathing. Skin contact matters less, but it should never be ignored, especially with solvents or additives that help spread the substance. Swallowing these compounds may set off gut trouble, like nausea or stomach ache, but inhaling the dust raises more serious health flags.
Vanadium isooctanoate doesn’t show up in research nearly as often as vanadium pentoxide. Still, its similarities ring the same alarm bells for those around the production and use of the chemical. If heated or broken down, it could release fumes that carry similar risks.
I’ve met workers in chemical plants who struggle to get straight answers about what’s dangerous in their day-to-day. The presence of chemicals like vanadium isooctanoate isn’t obvious—they could be in the pigments in a paint line or in drum storage for months. For those folks, risk isn’t an abstract, far-off thing. It’s about not wanting a cough to turn chronic, or to take trouble home. One guy I knew worked without a proper mask for weeks and ended up seeing a doctor because he couldn’t shake a heavy chest. Even small exposures made life uncomfortable.
Clear labeling is key. Workers need to know what’s in the drums or on their paint brushes before they open anything. Training staff about chemical hazards and making sure they use good masks and gloves isn’t just about rule-following; it means fewer sick days and a safer work life.
Ventilation plays a big role, especially in closed workshops or manufacturing spaces. Regular air quality checks catch leaks before health issues start. Industry needs regular reminders that swapping out old chemicals for safer alternatives pays off. If that’s not an option, sticking to safety data sheets and personal protection goes a long way.
Safety comes down to balancing efficiency and health. Companies and regulators have tools in place, but it takes real-world stories and clear facts to push for strong enforcement. No job is worth sacrificing your lungs or comfort, and that’s been proven time and time again on factory floors and construction sites.
Vanadium isooctanoate pops up in plenty of industrial settings, usually in catalysts and coatings. The thing is, this stuff carries certain risks, especially if folks get careless with storage. Having handled transition metal compounds in grad school labs, I learned to treat organometallics with extra caution. Sloppy storage doesn't just hazard a messy cleanup, it can mean a bigger health or fire risk.
Leaving vanadium isooctanoate exposed to humidity isn’t a minor mistake. Moisture likes to sneak in and spark up hydrolysis, which can break down the compound and sometimes create acids or oxides. That brings two headaches: unpredictable reactivity and possible damage to containers. Keeping things bone dry works. Good labs depend on desiccators or use sealed bottles with sturdy caps.
I always remember lectures warning against letting sensitive chemicals bake in the sun or freeze out in a truck overnight. Vanadium isooctanoate handles normal room temperature, but wild swings risk shorter shelf life or increased risk of breakdown. Direct sunlight can trigger changes too. Stashing it in a cool, dark place avoids these problems. Never store chemicals near radiators, open windows, or under heat lamps.
Glass beats plastic for storing organometallics, unless the substance wants to eat through the container. Vanadium isooctanoate tends to play fine with glass, but never trust a bottle with a dodgy seal or any sign of stress cracking. In companies I’ve worked with, techs always check inventory for cracked stoppers and swap out aging containers. Labels matter, too — clear, big writing, and hazard pictograms, not just faded ink and guesswork.
Factories and research spaces fix a lot of headaches by having good ventilation. Fumes build up quickly, especially from open bottles or accidental spills. Long-term exposure to vanadium compounds can irritate skin, nose, and lungs. Flammable vapors pump up the danger. Fume hoods save the day, as does keeping bottles tightly closed. I’ve seen coworkers come down with headaches after ignoring these habits.
The best teams I’ve worked with always knew where to find spill kits, absorbent pads, and emergency showers. It becomes routine to check eyewash stations and make sure chemical spill guidelines are posted in plain sight. Vanadium isooctanoate can be messy if tipped over, so readiness matters. Wearing gloves and goggles (not just safety glasses) gives extra protection. No one wants to scramble for gear after an accident has already happened.
Vanadium isooctanoate won’t take care of itself. Store it in a dry, cool spot, in solid glass bottles with no sketchy seals. Keep storage areas well ventilated. Always label everything clearly, check containers for damage, and prep for the unexpected. These simple steps keep people safe, products stable, and work environments healthy. Lab veterans know it only takes one careless mistake to trigger a problem that lasts the whole shift—or worse, longer.
Vanadium isooctanoate draws attention with a rich, dark liquid form that does not blend well with water. Instead, its oily nature keeps it more comfortable with organic solvents. Its color, usually deep amber to brown, hints at the presence of vanadium, a transition metal known for its vibrant oxidation states. The scent tells a story too: a sharp, somewhat pungent odor that professionals recognize in the lab.
Density falls between 0.9 to 1.2 grams per cubic centimeter, typical for metal carboxylates. The viscosity, often higher than that of simple oils, reflects the size and the branched structure of the isooctanoate ligand. This feature plays directly into how it's measured, stored, and transported—anyone working with it needs good ventilation and the right protective equipment. I still remember my early days handling similar compounds and learning to respect that slick, persistent residue that lingers on almost any surface.
Chemically, vanadium isooctanoate carries vanadium atoms bound to isooctanoic acid ligands. These organic “arms” stabilize vanadium in solution, making the metal easier to handle compared to many simple vanadium salts. Usually, the vanadium holds a +4 oxidation state, conferring deep reactivity but moderate air stability under proper containment—left open, exposure to air slowly converts some to the +5 state, altering its behavior and sometimes diminishing effectiveness in catalytic roles.
Solubility matters a lot. Vanadium isooctanoate mixes well with hydrocarbon oils and many polar aprotic organic solvents, such as toluene or xylene. This lets it play a central part in industrial blends, whether in coatings, inks, or as a catalyst in fine chemical synthesis. Unlike vanadium pentoxide—which is much less friendly to organic systems—this liquid salt dissolves without fuss and spreads its activity evenly.
Thermal sensitivity cannot be ignored. This compound does not hold up under open flames or prolonged high heat. At elevated temperatures (above 180°C), it starts decomposing, releasing irritating vapors and sometimes vanadium oxides. That means anyone working with it should treat it with the respect given to any potent catalyst: heating in a closed system, careful temperature control, and solid engineering safety practices.
In the real world, these physical and chemical traits shape the value and risks of vanadium isooctanoate. Its ability to stabilize reactive vanadium helps extend shelf life and simplifies shipping. At the same time, the oily, dense nature makes cleanup an extra challenge, demanding thoughtful waste management and spill response. I’ve found that even small spills become persistent if not mopped up immediately, often needing both solvent washes and absorbent powders.
This compound’s reactivity underpins its main use as a catalyst in polymerization, coatings, and alkene oxidation. The precise mixture of volatility, solubility, and thermal profile suits manufacturers aiming to control reaction rates and product qualities. But this same reactivity requires reliable containment; leaks lead to occupational hazards and environmental waste. In every lab or plant I’ve worked in, robust protocols and regular training kept incidents rare.
Safer processes mark the path forward. Field experience and published toxicity reports both point to vanadium compounds’ risk of lung and skin irritation, especially in chronic or careless exposure. Modern facilities now lean harder on sealed pumping systems and online sensors to catch leaks before they grow. Investment in staff training, rigorous hazard assessments, and secondary containment systems aligns well with the evidence and regulatory guidelines.
Even as new ligands challenge isooctanoate for top billing in modern processes, its unique blend of stability and reactivity earns it a continued spot in specialty manufacturing. If industry keeps refining both use and safety systems, vanadium isooctanoate will keep delivering value without sacrificing health and environmental standards.
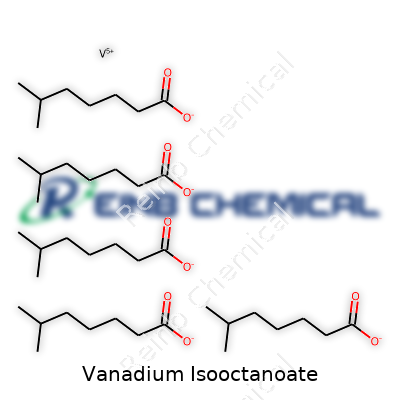
| Names | |
| Preferred IUPAC name | 2-ethylhexanoic acid; vanadium(3+) |
| Other names |
Vanadium 2-ethylhexanoate Vanadium octoate Vanadium(III) 2-ethylhexanoate |
| Pronunciation | /vəˈneɪdiəm aɪsəʊˈɒktə.noʊ.eɪt/ |
| Identifiers | |
| CAS Number | 97483-12-6 |
| Beilstein Reference | 1206807 |
| ChEBI | CHEBI:91175 |
| ChEMBL | CHEMBL4296741 |
| ChemSpider | 85827429 |
| DrugBank | DB01370 |
| ECHA InfoCard | The ECHA InfoCard of Vanadium Isooctanoate is **"100.249.096"**. |
| EC Number | 25759-14-0 |
| Gmelin Reference | Gmelin Reference: **Vanadium,624** |
| KEGG | C18627 |
| MeSH | D000077245 |
| PubChem CID | 101974542 |
| RTECS number | YM4550000 |
| UNII | I73C3SPA2X |
| UN number | UN3334 |
| CompTox Dashboard (EPA) | DTXSID60895962 |
| Properties | |
| Chemical formula | C24H51O4V |
| Molar mass | 483.55 g/mol |
| Appearance | Red brown liquid |
| Odor | Characteristic |
| Density | 0.98 g/cm3 |
| Solubility in water | Insoluble in water |
| log P | 3.3 |
| Vapor pressure | Negligible |
| Acidity (pKa) | 13.2 |
| Basicity (pKb) | pKb ≈ 3.0 |
| Magnetic susceptibility (χ) | -1.1e-6 |
| Refractive index (nD) | 1.490 |
| Viscosity | 12 - 20 mm²/s (40°C) |
| Dipole moment | 2.89 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | Std molar entropy (S⦵298) of Vanadium Isooctanoate is 748.6 J·mol⁻¹·K⁻¹ |
| Pharmacology | |
| ATC code | V10AX03 |
| Hazards | |
| GHS labelling | GHS02, GHS07, GHS08 |
| Pictograms | GHS07,GHS08 |
| Signal word | Warning |
| Hazard statements | H226, H302, H373 |
| Precautionary statements | P261, P264, P271, P272, P280, P301+P312, P330, P363, P304+P340, P312, P305+P351+P338, P337+P313, P370+P378, P403+P233, P405, P501 |
| NFPA 704 (fire diamond) | 2-1-0 |
| Flash point | Flash point: >110°C |
| Lethal dose or concentration | LD₅₀ (oral, rat) > 2000 mg/kg |
| LD50 (median dose) | LD50 (median dose): >5,000 mg/kg (oral, rat) |
| NIOSH | WA8370000 |
| PEL (Permissible) | 0.05 mg/m3 |
| REL (Recommended) | 0.1 – 1.0 phr |
| IDLH (Immediate danger) | IDLH: Not established |
| Related compounds | |
| Related compounds |
Vanadyl acetylacetonate Vanadyl naphthenate Vanadyl oxytriisopropoxide Vanadyl sulfate Ammonium metavanadate Vanadium pentoxide Vanadium(III) chloride |