
Chemistry tends to walk a line between old habits and bold new risks. Diphenylisooctyl phosphite started gaining attention in the late 20th century, in a decade when polymer innovation sprinted forward. Plasticizers, stabilizers, and antioxidants all came out of a need to keep plastics useful under sunlight, heat, and time, and each compound added to the toolbox changed the industry. Chemists didn’t just happen across this substance; decades of phosphite research drilled down into the fine details of molecular structure, coming up with new side chains that made the additive more compatible with different polymer matrices. Early trials focused on triaryl and trialkyl phosphites, but blending bulky isooctyl groups with diphenyl moieties landed on a solution that fit the performance gap between volatility and efficiency.
Anyone who has worked with Diphenylisooctyl phosphite recognizes it by its thin, oily liquid appearance, usually faint yellow. It's a phosphite ester, made by attaching phenyl and isooctyl groups to a phosphorus center. Markets classify it under polymer stabilizers—its main job is to shield plastics, especially PVC, from heat-induced degradation during processing and service life. This isn’t just another commodity chemical; some manufacturers use product codes like PIOP, DPIOP, or list it as phosphorous acid, isooctyl diphenyl ester. The market now demands high purity formulations, so suppliers reference color, refractive index, and phosphorus content on the certificate of analysis.
With a molecular formula of C28H39O3P, Diphenylisooctyl phosphite weighs in at about 454 g/mol. Its boiling point passes 250°C under reduced pressure, which helps prevent volatilization in most PVC extrusion lines. It stays fluid at room temperature, with a density around 1.0–1.02 g/cm3. It barely dissolves in water, but it mixes well with most nonpolar solvents, including phthalate plasticizers and chlorinated hydrocarbons regularly used in PVC compounding. One key chemical trait: it works as a reducing agent. That means it scavenges free radicals during thermal processing, putting out the fires before they turn polymers brittle or discolored. Slight hydrolysis risk comes from storage in wet conditions—a slow breakdown forms phenol and isooctanol, both irritants but well-understood in the industry.
Industry standards for Diphenylisooctyl phosphite usually revolve around purity over 98%, phosphorus content not less than 5.5%, and maximum color index (APHA) below 50 for top grade materials. Suppliers must declare moisture content—below 0.1% gives the best storage performance. The product label displays the UN number for hazardous goods, shipping class (for example, 9 for miscellaneous dangerous substances), and a hazard pictogram per GHS. Technical datasheets don't just reference shelf life—they include recommended dosage levels, compatibility tables with phthalate or non-phthalate systems, and sometimes troubleshooting guides for extruder operators facing yellowing or plate-out issues.
Manufacturing Diphenylisooctyl phosphite calls for careful batch chemistry. The process starts with phosphorus trichloride, bringing in phenol under controlled, cooled conditions, forming diphenyl phosphite with hydrogen chloride as the byproduct. The intermediate reacts with isooctanol, sometimes under nitrogen to avoid air oxidation. Catalysts or acid scavengers help keep side-reactions from chewing up yield, which helps the economics. Chemical engineers worry about removing color bodies and leftover acids—so vacuum distillation and scrubbing are standard steps before final filtration. Lab tests on the batch check for unreacted alcohol and acid number to make sure the product won’t corrode metal or trigger side reactions in the finished application.
The big selling point for Diphenylisooctyl phosphite is its behavior under heat—it steps up as an antioxidant, catching peroxides before they break apart a polymer chain. Under typical extrusion conditions, it reacts with trace peroxides formed during compounding, releasing secondary alcohols and oxidized phosphorus acids, most of which stay inert in the matrix. Blending with other phosphites or metal soaps can adjust melt processing performance, improve transparency, or prevent exudation on cable insulation. Chemists sometimes tweak the isooctyl group to octyl or nonyl variants, aiming for even lower volatility or better plasticizer compatibility.
Depending on the supplier or patent, Diphenylisooctyl phosphite goes by different trade names: iso-Octyl diphenyl phosphite, DIPO, or specific catalog codes like Santicizer 141. Sometimes it’s listed in technical documents as phosphorous acid, isooctyl diphenyl ester, just to trip up anyone searching for a safety sheet. These differences matter mainly for procurement—and anyone who has ever scrambled for REACH or TSCA approvals knows the hassle of tracking down the right substance under twenty different registry listings.
Safety teams in chemical plants spend time updating operational guidelines. Diphenylisooctyl phosphite can cause skin and eye irritation and must not be inhaled as an aerosol. Good industrial hygiene means operators always suit up with gloves, goggles, and chemical-resistant aprons before handling bulk containers. Labs run regular monitoring for air emissions because thermal decomposition emits toxic phosphorous oxides. Storage best practice: keep containers in a dry, well-ventilated warehouse, away from strong oxidizers. Spill protocols emphasize containment and disposal under local hazardous waste rules. Shipping regulations enforce strict labeling, especially for export—something every compliance manager grapples with during annual audits.
PVC processing plants have long relied on phosphite stabilizers for reasons beyond simple economics. Under the heat of extrusion or calendaring, vinyl chains break down if not protected. Diphenylisooctyl phosphite helps keep flexible films, insulation, and coated fabrics looking clear and strong, without yellowing or cracking after months under sunlight. Cable manufacturers add it to insulation compounds for heat stability and flexibility since electronics can’t afford brittle jackets. Other plastics—from polyolefins to styrenics—also see benefits in specific blends. Secondary uses have popped up in adhesives, synthetic rubber formulations, and coatings where long-term clarity beats out other stabilizer choices.
In the last decade, funding has shifted towards more eco-friendly and high-efficiency stabilizers. Research labs run performance testing on new variants, blending molecular modeling with accelerated aging studies in real polymer matrices. Some teams look at using renewable alcohols as feedstocks for the isooctyl group, either from bio-based butanol or from catalytic transformations of plant oils. Lab studies focus on lowering migration rates, aiming for food contact safety while holding onto processing stability. Academic groups dig deeper into thermal degradation pathways, using NMR and GC-MS to trace breakdown products and optimize stabilizer lifetime. Patent filings keep climbing as manufacturers race to pin down proprietary blends and new process improvements.
Toxicologists always dig into the byproducts as much as the main chemical. Acute toxicity for Diphenylisooctyl phosphite stays low—rodent studies set oral LD50 levels at several grams per kilogram. The main concern links to slow hydrolysis over time, which sheds phenol and isooctanol in trace amounts. Chronic exposure limits focus more on safe handling in industrial settings than consumer end-use, and agencies like ECHA and EPA monitor new findings. In most polymer applications, the stabilizer gets locked into the matrix, limiting migration or exposure. Yet, regulatory trends push for ever-stricter migration studies, especially for anything used in food packaging or children’s toys.
Pressure to move away from heavy metal stabilizers gives Diphenylisooctyl phosphite a field of opportunity. Manufacturers pick it for modern compounds aiming at RoHS and REACH compliance. Advances in polymer technology now look for stabilizer systems tailored for recyclability and low environmental impact—here, phosphites like this one show promise if researchers can further cut down migration and environmental footprint. Companies large and small invest in closed-loop manufacturing, and drop-in stabilizers fit existing equipment, opening up the market for technical upgrades without complete re-engineering. Demand grows fastest in regions upgrading infrastructure and switching to safer chemical regimes, putting this compound near the forefront of responsible manufacturing in the global plastics sector.
Diphenylisooctyl phosphite isn’t the kind of chemical name that rolls off the tongue. Still, its role plays out in products most of us touch every day. This phosphite functions mainly as a stabilizer in the production of plastics, with a particular focus on PVC (polyvinyl chloride). PVC shows up in shower curtains, cables, pipes, and even some children’s toys. Without a stabilizer like diphenylisooctyl phosphite, plastics risk breaking down much earlier—from heat, light, or the constant push and pull of daily use.
Very few people follow the journey of raw plastic from factory to living room, but it’s a messy ride full of heat, pressure, and chemical mixing. As plastics mold into shape, they pick up stress. That’s where stabilizers step in. Diphenylisooctyl phosphite acts as a kind of shield against oxygen and heat, slowing down the process that turns tough plastic brittle and yellow. Chemists call this “oxidative degradation.”
My own time in a plastics facility opened my eyes to this. We ran trial batches with and without extra stabilizers. Without enough phosphite, the plastic sheets would crack after a few weeks of sunlight, ruining an entire shipment. After that, the team made daily checks just to avoid a costly repeat.
It’s only fair to wonder whether a chemical that prevents plastic from falling apart sticks around where it shouldn’t. The key is dosage and regulation. Agencies such as the European Food Safety Authority have investigated the chemical. Most uses pass strict limits to keep it from leaching out at dangerous levels. That benefits public health, but it also puts pressure on manufacturers to monitor batches and stick to safe processes.
Industry standards set maximum allowable amounts, not because manufacturers like jumping through hoops, but because history has taught us about overlooked risks—think phthalates in teething rings or migration from plastic wrap into food. Continuous research helps keep bad surprises out of kitchens and playgrounds.
Questions crop up about what happens to these additives once a product hits the landfill. Plastics outlive plenty of humans, after all. Some of these stabilizers break down very slowly, if at all. Studies suggest traces show up in the water or soil over time. That leads to bigger environmental worries, especially as microplastics collect in ecosystems across the planet.
Change never comes overnight, but the plastics industry stands at a crossroads. Scientists keep searching for safer, degradable additives. I’ve seen university labs take an interest in how these chemicals behave in compost, trying to speed up natural decay or swap out formulas for safer ones. Calls for stronger recycling standards keep growing. Companies respond by tweaking formulas and investing in closed-loop recycling programs that cut pollution at the source.
Diphenylisooctyl phosphite remains popular because it works. Yet the conversation keeps circling back to safety and the planet’s future. The right move involves transparency, research, and pushing hard for green chemistry that can do the job without adding to tomorrow’s cleanup list.
Working around industrial chemicals gets personal quickly. Years ago, I spent my summers in a plastics lab and came to respect how chemicals like Diphenylisooctyl Phosphite—often called DIOP among chemists—can seem harmless in a beaker but pack health concerns nobody should overlook. It’s easy to see a clear liquid and forget what’s carried in its invisible fumes, or what clings to your gloves at the end of a shift.
DIOP plays its part in stabilizing plastics, especially when heat threatens to break down materials. Companies appreciate its behind-the-scenes benefits, but research flags several points about safety. According to globally recognized resources like the U.S. National Institute for Occupational Safety and Health (NIOSH) and studies cited by the European Chemicals Agency, DIOP can irritate skin, eyes, and respiratory pathways. Repeated or long-term exposure potentially causes more severe issues, including harm to organs like the liver.
There’s a reason regulatory bodies require labeling for compounds like this: People have ended up with chemical burns or chronic coughs after unprotected handling. The workplace stories get even more serious when accidental spills or vapor exposure aren’t addressed right away. I’ve seen skilled technicians shrug off their masks for five minutes, only to regret it as headaches set in a little later. Personal protective equipment and ventilation aren’t optional extras—they draw a clear line between a safe environment and a silent health risk.
Safety data sheets add itemized lists of risks and suggest gloves, goggles, and respirators for a reason. The risk doesn’t vanish just because a liquid sits still in a bottle. Routine accidents, from splashes to broken packaging, show up in incident logs year after year. DIOP’s low odor and color trick senses into thinking danger isn’t present, but lab analysis confirms inhalation or skin contact carries risks over weeks or months, not only in single exposures.
People managing chemicals get better results when they handle DIOP with respect at every stage—moving, measuring, even storing. Closed systems, regular air quality checks, and training sessions for new staff raise safety across the board. Simple habits—washing hands thoroughly after use, changing gloves between tasks, keeping containers sealed—make a noticeable impact on whether technicians stay healthy over a career.
A culture of safety goes a long way. Every lab and factory needs upfront investment in the right gear, practical ventilation, and frank conversations about risks. Managers should stay up to date with new studies, since industry understanding of chemical impacts can shift with new research. Staff ought to report even small symptoms—skin irritation, unusual fatigue, headaches—so workplace safety doesn’t slip through the cracks.
From my time in the field, the safest teams pay attention to the little stuff. Daily checks, clear instructions, and a no-shortcuts mentality protect not only individual health but also peace of mind for families waiting at home. The tech making your car lighter or your smartphone shell tougher relies on people not cutting corners with DIOP and other phosphites.
Safe handling of DIOP—like so many synthetic chemicals—starts with knowledge and builds with habit. Pushing for safer alternatives and better protective systems remains every worker’s right. The value found in chemical tools never outweighs the cost of a carelessly handled accident or a long-term health problem that could have been prevented.
Diphenylisooctyl phosphite plays a quiet but crucial part in many industries, especially plastics and rubber manufacturing. If you've spent any time in a plant or warehouse that handles chemical additives, you probably know how easy it is for things to go wrong if people take shortcuts in storage. Chemicals like this seem stable, but only as long as conditions stay right. I’ve watched a warehouse manager groan at labels peeling off metal drums and seen what poor planning can do: unexpected reactions, weird odors, ruined material, and sometimes real dangers to people working nearby. That sort of thing leaves a mark on your memory and shapes how you approach chemical safety.
One important requirement for storing diphenylisooctyl phosphite is limiting its exposure to air and moisture. This chemical can react with water in ways that aren’t just a problem for quality control. Hydrolysis leads to the formation of corrosive byproducts, which can eat away at metal containers and create leaks or spills. In my own experience, storing drums in a part of the facility with leaky roofing once led to a sticky mess in a quiet corner nobody visited. It took a weekend to clean up, and it cost more than just downtime. That moment taught the crew just how important dry, sealed containers really are.
Heat is another real troublemaker. Prolonged exposure to higher temperatures boosts the risk of degradation and sometimes even dangerous chemical reactions. I’ve seen storage rooms go above 30°C during peak summer afternoons. For sensitive chemicals, that’s never good news. Dedicating a shaded, well-ventilated section of your facility can make a big difference. Cooling solutions don’t have to be high-tech; sometimes a well-placed fan and insulation stop temperatures from climbing. If local regulations call for fireproof storage—there’s a reason for that. Phosphite compounds can give off toxic fumes during a fire. If you’ve ever had to review a safety drill, you know how much depends on easy access to information and clear walkways, especially when the wrong reaction could fill a room with harmful vapors.
Not all containers handle diphenylisooctyl phosphite equally. In my time around warehouses, steel drums lined with plastic carried this chemical more safely than unlined barrels, thanks to the corrosive potential of phosphites and their byproducts. Make sure every drum, tote, or container carries up-to-date hazard labeling and seals tightly—old inventory with cracked gaskets is a disaster in the making. Keeping rigorous inventory checks and following a first-in, first-out approach reduces the odds of finding an unmarked, mystery container buried in a back corner.
I’ve watched teams cut corners to save a few minutes, only to spend hours later dealing with chemical leaks. It isn't just about telling people the rules. Supervisors do much better by training their staff to actually understand these risks, backing it up with checklists and regular inspections. Whenever a container arrives or leaves storage, have a process to scan labels and confirm seals. Never skip the step where you write down when something goes in or out.
No one wants to face an audit and realize a chemical’s been sitting too close to a heat duct or stacked next to something incompatible. Over the years, I’ve seen that thoughtful storage is about attention to detail: keeping diphenylisooctyl phosphite out of the sun, away from water, under lock and key, and marked clearly so there’s no question what’s inside. Careful planning, honest training, and routine checks go a long way to protecting both workers and investments.
In the field of industrial chemistry, knowing the makeup of specific additives matters. Take Diphenylisooctyl Phosphite, for example. Its chemical formula is C20H35O3P. This combination of carbon, hydrogen, oxygen, and phosphorus plays a big role in how the substance acts and what it does in products—especially plastics and rubbers. People working with stabilizers often come across this compound. That formula isn’t just a string of letters and numbers, it marks out every atom in each molecule and shapes how it behaves under tough conditions like heat or exposure to oxygen.
A well-understood chemical formula matters for two key reasons: performance and safety. Folks who make PVC pipes or vinyl flooring find out very quickly that additives make or break the end result. In my experience, you learn to respect these numbers. That “C20” and “H35” aren’t just statistics, they mean Diphenylisooctyl Phosphite packs long, bulky hydrocarbon chains. Those chains let it fend off reactions that would shorten the life of the material. The presence of phosphorus sets it apart as an antioxidant, tackling the free radicals that break down polymers. It’s not magic—just smart chemistry.
Diphenylisooctyl Phosphite doesn’t stay behind-the-scenes forever. The compounds you rely on in manufacturing, agriculture, and packaging often spend time with it. Because of that, knowing the formula is more than a lab curiosity—it shapes the safety rules, handling procedures, and even the environmental plans companies follow. For instance, improper handling could lead to phosphite breakdown in sunlight, which has environmental impacts. Those working with materials containing this additive depend on ECC (expertise, experience, and confidence) to judge storage temperatures or mixing partners. That all starts by understanding what’s in the bottle.
Admitting the truth: no additive is perfect. Phosphite stabilizers serve well, but health authorities have eyes on every substance that touches food packaging or enters waste streams, including Diphenylisooctyl Phosphite. Some byproducts or breakdown components could raise eyebrows in regulatory reviews, which demands rigorous testing and transparent supply chains. Manufacturers and regulators both gain from knowing the chemical structure: the right formula lets labs predict reactivity and guide smarter recycling or disposal methods. In the industry, this pushes chemists—myself included—to keep one eye on that chemical formula as they tweak components for better safety or easier traceability.
As materials science moves forward, success depends on getting the basics correct. A small change in a compound’s molecular formula shifts its whole story. For newcomers learning the ropes or companies adapting to new regulations, accuracy in these details proves vital. That attention to formulation—right down to “C20H35O3P”—builds a foundation for safer products, better regulations, and less guesswork on the factory floor. It keeps the science grounded, the safeguards current, and the learning continuous.
Every time I walk through a warehouse or lab stocked with industrial chemicals, I think about the long trail each one leaves behind. It’s not just about storing them or even using them safely. Sooner or later, the question turns to disposal. With chemicals like Diphenylisooctyl Phosphite, there’s more at stake than just emptying a drum and moving on to the next task. Contaminated water or polluted air risks real health and environmental harm. Plenty of folks overlook disposal until they hit a wall or run into inspections. Having worked in both small labs and bigger industrial setups, I’ve seen that doing things the right way from the start saves a lot of headaches and keeps communities safer.
This chemical turns up in plastics and as a stabilizer in the vinyl industry. Left unchecked, it can break down into compounds that stick around in soil and water. We're not just talking about extra work for environmental cleanup crews—people or wildlife can end up exposed through water supplies and air. According to the European Chemicals Agency, phosphite-based additives show persistence and possible bioaccumulation. I've seen regulators get tough after noticing traces pop up in water testing. So what feels like a simple misstep on the factory floor can spiral into fines, lawsuits, and long-term costs.
The safest route runs straight through specialized hazardous waste treatment. Landfills built for regular trash won’t cut it. I’ve watched companies partner with licensed hazardous waste handlers who collect the chemical, label containers clearly, and use sealed transportation to designated facilities. Their teams wear proper protective equipment—because even a splash or fume leak can cause health issues. Incineration at high temperature, in facilities equipped to handle organophosphorus materials, gets mentioned often in guidance issued by the EPA and international bodies. High heat breaks down the compound into simple gases that scrubbers catch before anything releases into the atmosphere.
I’ve seen firsthand that reducing waste beats managing a big stockpile of leftovers. Tighter inventory controls, using up older materials first, and training staff to avoid spills all make a difference. Using just-in-time delivery can help prevent chemicals from sitting around and degrading. If a spill happens, absorbent pads and neutralizers stop it from running into drains. Documentation—something a lot of folks skip—protects both the workers and the company. Logs of what’s produced, stored, and disposed of often settle disputes and clear up confusion during audits.
Different regions set their own rules for handling chemicals. Those regulations exist for good reason. Following local hazardous waste codes lowers risks and keeps operations running smoothly. Local community members also have a right to know about the chemicals moving through their neighborhoods. Public databases, transparent reporting, and open lines for community questions help build trust. In my experience, companies that talk openly about their disposal processes end up with fewer complaints and better working relationships across the board.
No one loves regulatory paperwork, but compliance sets a standard that benefits workers and neighbors alike. Ongoing education, routine facility inspections, and open conversations with disposal partners keep things moving in the right direction. There’s always pressure to cut costs, but proper disposal pays dividends in peace of mind and public safety. Diphenylisooctyl Phosphite won’t go away anytime soon, but with some effort and attention, its risks can stay under control.
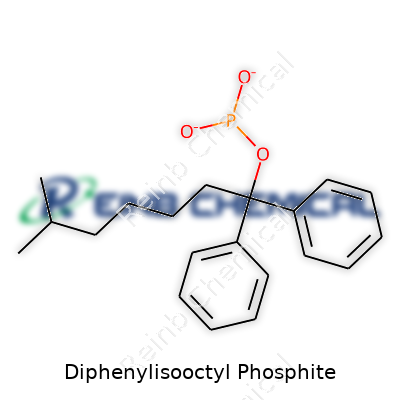
| Names | |
| Preferred IUPAC name | 2-Ethylhexyl diphenyl phosphonite |
| Other names |
Phosphorous acid, isooctyl diphenyl ester Phosphorous acid, isooctylphenyl diphenyl ester Isooktyldifenylfosfit Fosforous acid isooctyl diphenyl ester |
| Pronunciation | /daɪˌfiːnɪlˌaɪsoʊˈɒktɪl ˈfɒsfaɪt/ |
| Identifiers | |
| CAS Number | 18045-86-6 |
| 3D model (JSmol) | `3Dmol:'C1=CC=CC=C1OP(OC(C)(CCCCCC)C2=CC=CC=C2)(OC(C)(CCCCCC)C2=CC=CC=C2)=O'` |
| Beilstein Reference | 1241480 |
| ChEBI | CHEBI:34717 |
| ChEMBL | CHEMBL1377276 |
| ChemSpider | 13342422 |
| DrugBank | DB16644 |
| ECHA InfoCard | ECHA InfoCard: 100.048.318 |
| EC Number | 262-898-6 |
| Gmelin Reference | 1525133 |
| KEGG | C16512 |
| MeSH | D008945 |
| PubChem CID | 20936 |
| RTECS number | TD0350000 |
| UNII | 561346Y4X9 |
| UN number | UN3272 |
| CompTox Dashboard (EPA) | DTXSID5050897 |
| Properties | |
| Chemical formula | C20H27O3P |
| Molar mass | 502.62 g/mol |
| Appearance | Colorless transparent liquid |
| Odor | Odorless |
| Density | 0.99 g/cm3 |
| Solubility in water | Insoluble |
| log P | 7.74 |
| Vapor pressure | <0.01 mmHg (25°C) |
| Acidity (pKa) | 13.1 |
| Basicity (pKb) | 6.10 (pKb) |
| Magnetic susceptibility (χ) | -1115.0 × 10^-6 cm³/mol |
| Refractive index (nD) | 1.5230 |
| Viscosity | 230 mPa.s at 25°C |
| Dipole moment | 3.07 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 492.6 J·mol⁻¹·K⁻¹ |
| Std enthalpy of combustion (ΔcH⦵298) | -10835.8 kJ/mol |
| Hazards | |
| GHS labelling | GHS02, GHS07 |
| Pictograms | GHS07,GHS09 |
| Signal word | Warning |
| Hazard statements | H302, H315, H319, H411 |
| Precautionary statements | P210, P260, P273, P280, P301+P312, P305+P351+P338, P308+P313, P501 |
| NFPA 704 (fire diamond) | 1-2-0-Health: 1, Flammability: 2, Instability: 0 |
| Flash point | 210°C |
| Autoignition temperature | Autoignition temperature: 445°C |
| Lethal dose or concentration | LD50 (oral, rat): > 25,000 mg/kg |
| LD50 (median dose) | LD50 (median dose): Rat oral 7 g/kg |
| PEL (Permissible) | Not established |
| REL (Recommended) | 0.1 mg/m³ |
| Related compounds | |
| Related compounds |
Triphenyl phosphite Tris(2-ethylhexyl) phosphite Diphenylcresyl phosphite Tris(nonylphenyl) phosphite Triphenyl phosphate |