
2-Ethylhexanoic acid traces its story back to the early twentieth century when chemists looked for carboxylic acids with enough versatility to carve a place in a growing world of advanced industrial formulations. Early efforts focused on deriving functional acids from oxo-alcohols, particularly as plasticizers and metal salt precursors. As the chemical industry boomed—especially after the Second World War—demand for such functionalized acids shot up. Before modern synthetic routes took hold, extraction and separation stayed laborious, but process engineers pressed forward, eventually cracking the code for commercial production. The post-war era saw this chemical gain traction, laying groundwork for today’s widespread applications in plastics, coatings, and lubricants. The historical development of 2-ethylhexanoic acid stands as a mirror, reflecting changing market needs, better catalyst systems, and expanded regulatory oversight, all the way from the simple aldehyde building blocks to complex downstream products.
This molecule belongs to the class of carboxylic acids. In my years working with specialty chemicals, I’ve seen how its branching structure sets it apart, offering more solubility in organic solvents and less volatility than lower analogues. It often ends up as a precursor for metal soaps or as an intermediate in component libraries for formulations. Industrial plants tap its usefulness in synthesis rather than as a consumer-facing chemical. It often finds its way into markets through grades marked by purity, color, and trace impurity thresholds, impacting everything from yield in reactors to quality outcomes in plastics and lubricants. In commercial settings, buyers scrutinize batch-to-batch consistency, given that even minor impurities can throw off downstream metal complexations or pigment preparations.
With molecular formula C8H16O2, 2-ethylhexanoic acid appears as a colorless to pale yellow liquid, slightly oily to the touch, and bearing a faint acrid odor. Its boiling point hovers around 228–232°C, which fits applications demanding thermal stability above what short-chained acids can handle. It weighs in at about 144.21 g/mol and shows moderate solubility in water but dissolves easily in alcohols, ethers, and other polar organic solvents. The branched-chain structure dulls the crystalline behavior typically seen with linear carboxylic acids, keeping it a liquid under most environmental conditions. A pKa value of around 4.88 signals moderate acidity, making it a strong enough acid for forming stable salts with transition metals, yet gentle enough for polymerization and coating processes.
Those working in procurement know that specification sheets form the backbone of each purchase of 2-ethylhexanoic acid. Purity usually hits at least 99% by GC, with low water content and strict limits on aldehydes and other carboxylic acids. Product is often shipped in lined drums or tankers, with each container labeled according to international transport and GHS protocols. Each drum carries hazard statements and pictograms, warning of skin and eye irritation risks with long-term exposure. Suppliers also track batch numbers and COA (Certificate of Analysis) data for traceability and regulatory compliance. Barcodes and QR codes have become common sights on labels, giving logistics teams easy access to digital traceability and quality control logs.
Industrial production pivots on the catalytic oxidation of 2-ethylhexanal, an aldehyde derived itself from the oxo process using propylene and synthesis gas followed by hydrogenation. Chemical engineers often prefer air oxidation in the presence of catalysts—cobalt or manganese salts come up often here—to push conversion rates up and control byproducts. Temperature and pressure adjustments help operators nudge yields higher while minimizing over-oxidation, which chews through profits by creating unwanted byproducts. In research labs, people sometimes try direct carboxylation or alternative aldehyde sources, particularly for radio-labeling studies. Most industrial facilities monitor process streams in real time, keeping tabs on reactor ratios, heat flow, and phase separations to meet cost targets and reduce waste streams. Sodium and potassium 2-ethylhexanoates spring up easily from neutralization reactions, feeding downstream producers in the drier, solubilized forms they prefer.
2-Ethylhexanoic acid’s carboxyl group opens the door for esterification, salt formation, acylation, and polymer modifications. People rely on ester products—think 2-ethylhexyl 2-ethylhexanoate in emollient-rich cosmetics or lubricants—for their smooth skin feel and oxidation resistance. Metal 2-ethylhexanoates, such as cobalt or zinc salts, play key roles as catalysts in paint driers or as stabilizers in PVC. Industrial installations frequently blend these salts for tailored performance in anti-corrosion and pigment-drying blends. Hydrogenation introduces more functional derivatives, but the acid’s bulkiness does limit some classic Friedel-Crafts acylations. Those who want to graft it onto polymers benefit from its improved compatibility and lower volatility compared to straight-chain analogues, enhancing the flexibility and weather-resistance of plastics in harsh climates.
The trade often calls it 2-ethylcaproic acid, but catalogues sometimes list it under octanoic acid, iso-octanoic acid (though this can cause confusion with the true isomers), or EHA. CAS number 149-57-5 keeps ordering unambiguous. Brands sometimes offer product lines with suffixes reflecting purity or added inhibitors. Distributors might list proprietary blends or stabilized solutions using in-house names, yet they reference the same molecular structure at heart, relying on international nomenclature for safety data sheets and trade compliance.
On the factory floor, 2-ethylhexanoic acid draws respect for its irritant effects. Long-term exposure—especially in poorly ventilated areas—may sensitize skin and respiratory passages. Safety data sheets warn of splashing and inhalation during weighing, pouring, and mixing, pushing best practices toward local exhaust ventilation and chemical-resistant gloves. Googling occupational health bulletins turns up exposure limits, but most companies shoot for far below mandated thresholds. Training programs teach workers about proper spill handling, safe container opening, and emergency wash procedures, with digital logbooks tracking each incident or near-miss. Regulators in Europe and North America track safe usage, especially as new toxicological data emerge and more drugs or plastics use this acid as a building block. As environmental rules tighten, the industry watches emissions and waste streams closely and invests in capture and treatment to curb releases into waterways.
If you walk through a modern chemical plant, you’ll see 2-ethylhexanoic acid in drums headed for coatings, lubricants, and polymer additives. Cobalt and manganese salts head off for use in alkyd paint driers, giving fast curing for wood lacquers and automotive enamels. Lubricant manufacturers dissolve it into synthetic base stocks, soaking up its low volatility and thermal stability for high-performance greases and motor oils. Automotive and electrical engineers value its derived esters for their role as plasticizers, helping soften plastics for wire and cable insulation. Cosmetics chemists exploit its emollient properties, blending it into creams and lotions for a non-tacky skin feel. The acid also pops up as an intermediate in pharmaceuticals, especially in prodrug synthesis for its ability to navigate metabolic pathways. Metallurgy labs count on its chelating ability for preparing metal catalysts, while electronics producers study its salts for use in vapor deposition and etching.
Research teams keep trying to squeeze more value from this molecule. Biodegradation pathways attract much attention, given pressure to push sustainability goals in the chemical sector. Alternative synthesis routes—using renewable feedstocks for the precursor aldehyde—sit at the front line in green chemistry journals. Catalysts based on rare earths offer some promise for higher selectivity, though cost remains a concern. There’s active exploration in modifying the acid’s structure to produce next-gen plasticizers that skip regulatory scrutiny. Multinational producers fight to minimize residual impurities, tracking trace substances in downstream consumer products, especially as more countries move toward stricter consumer protection laws. Pharmaceutical research keeps dissecting how alkyl-branched acids interact with metabolic enzymes, tweaking prodrug and delivery system designs for advanced therapies. R&D keeps focusing on tightening specs, boosting process safety, and unlocking new, value-added uses.
Toxicologists explore 2-ethylhexanoic acid’s metabolism, focusing on how the body handles branched-chain carboxylic acids. Animal studies show that repeated high-dose exposure can affect liver function and fetal development, drawing regulatory scrutiny in applications touching pharmaceuticals and personal care. Agencies such as the European Chemicals Agency (ECHA) flag it for reproductive toxicity, prompting some manufacturers to substitute or restrict its use in consumer goods. Many researchers tune analytical assays—mass spec, NMR, and metabolomics—looking for exact metabolic fates. Occupational health monitoring focuses on skin exposure, inhalation, and accidental ingestion, noting irritation as the main concern in day-to-day factory use. Industry groups sponsor chronic toxicity studies, feeding data into international reporting frameworks and creating more transparent safety dossiers that inform both formulation choices and public policy debates.
The outlook for 2-ethylhexanoic acid swings with trends in plastics, paints, and sustainability. The chemical industry faces mounting pressure to phase out substances with reproductive toxicity, especially in personal care and children’s products. That challenge puts R&D under the gun to find drop-in replacements or engineer safer derivatives without losing performance. Meanwhile, demand holds steady for industrial sectors where alternatives don’t measure up—especially coatings and synthetic lubricants. The shift toward renewable feedstocks pulls some focus to bio-based or waste-derived raw materials, leading pioneering plants to test greener processes. Digitalization and real-time analytics help refine process safety and efficiency, making it easier to squeeze out costly variances and reduce exposure risks. As regulatory regimes grow more complex worldwide, adaptability and transparency become a producer’s best tools for maintaining trust along supply chains. With smart innovation and an eye on occupational health and environmental impact, the chemical will continue finding a balance between utility and responsibility, shaping the next generation of industrial chemistry.
Many folks haven’t heard of 2-ethylhexanoic acid, but it’s been hiding behind the scenes in plenty of things. Take a walk through any hardware store, car shop, or you might even have it in some cleaning products at home. This simple compound shows up in places far more common than its tricky name would tell you.
In the auto industry, everyone wants their engine to last longer and break down less. Here, 2-ethylhexanoic acid does some heavy lifting. Used to create certain metal salts, it helps shield engines from corrosion. If you’ve ever checked out the price of car repairs due to rust or fluid leaks, you know the value of something that fights that chemical attack inside your radiator.
From my own days wrestling stubborn bolts under an old pickup, I’ve seen firsthand how neglected cooling systems turn into expensive headaches. The additives built from this acid strengthen antifreeze formulas, giving engines a longer shot at survival. If auto shops skipped on these kinds of ingredients, I bet there’d be a lot more overheated cars pulled over on the shoulder.
Paints and coatings are another place 2-ethylhexanoic acid makes a difference. It’s a key piece in preparing drying agents called “metal soaps.” Picture this: you roll a fresh coat of paint on wood or metal, and it needs to dry smooth and solid, not gummy or tacky. These soaps soak up oxygen fast, moving the drying process along. The finished surface looks better and lasts through more seasons. At home, dealing with trim or outdoor railings, I’d rather wait for paint to dry because it’s ready, not because it’s still sticky three days later.
Plastics take up more space in our lives each year. Soft toys, flexible hoses, floor tiles—they all depend on plasticizers to keep from going brittle. Some of these plasticizers come from 2-ethylhexanoic acid. Without them, playground balls crack, and vinyl siding would fall to pieces when the sun gets tough. Manufacturers lean on this compound to boost flexibility and keep consumer products looking and working as expected.
You wouldn’t guess it from reading a label, but cleaners and detergents sometimes depend on this acid. It teams up with metals to help break down stains and grime, especially on industrial machinery. I’ve spent afternoons scrubbing greasy gearboxes in a machine shop with specialized degreasers—and the mix worked because the chemistry was quietly doing what elbow grease and hot water can’t.
Not everything about 2-ethylhexanoic acid is rosy. There’s concern about its effect on health and the environment. Lab studies point to risks for both workers and wildlife when handled carelessly. Manufacturers must keep exposure under control, with protective gear and good ventilation. Regulatory bodies keep labs and factories honest here, with strict rules on limits and handling. These checks matter because shortcuts in safety turn into stories about hospital visits or long-term health issues.
Innovation and safety need to run side by side. Chemical companies can lean into better alternatives and tighter safety rules. Industry can make improvements in recycling and waste treatment. Speaking as a parent and a former mechanic, I think every layer of protection—from safer bottles at home to stricter factory inspections—counts. Making smart choices now will keep both workers and customers safer in the long haul, so this powerful compound can keep helping without causing harm.
2-Ethylhexanoic acid carries the chemical formula C8H16O2. That’s eight carbon atoms, sixteen hydrogens, and two oxygens. Take a closer look at the structure, and a story emerges: a carboxylic acid group (-COOH) sits at one end while the chain stretches out, branching at the second carbon due to an ethyl group. This branching forms a “fork” early in the chain, with the main backbone running for six carbons and the fork pointing out from carbon two. That branch—two carbons sticking out from near the start—gives it the “2-ethyl” name. The molecule finishes with a six-carbon tail, making the full chain eight carbons long, counting the fork.
Plenty of folks never hear about 2-ethylhexanoic acid unless they dive into chemistry labs, specialty plastics, or industrial lubricants. Its structure helps it dissolve in oils, mix with a wide range of molecules, and stabilize metal ions. This isn’t just chemical trivia. In my time working on surface coatings, I saw how critical these molecules become for making sure paint holds up against rain and sun. The acid finds its way into metal soaps used for drying paints, keeping everything from pipelines to phone poles looking sharp and rust-free.
You also find 2-ethylhexanoic acid in synthetic lubricants, coolants, and plasticizers. The forked structure delivers a mix of flexibility and strength—key traits when you need a substance to function at extreme temperatures or hold strong against chemical attacks. Medical fields use derivatives for certain drug carriers, while electronics industries rely on its metal salts for stabilizing components. The molecule’s shape and size influence performance at these jobs, acting a bit like a specialized wrench meant for just one kind of bolt.
Too often, the conversation about specialty chemicals skips over health or environmental impact. I’ve learned firsthand that this can’t get ignored. 2-ethylhexanoic acid isn’t heavily restricted, but persistent exposure can pose risks—think reproductive toxicity flagged by some studies. It’s not a household name like lead or mercury, but manufacturers who use it carry responsibility for air quality and wastewater discharge. The chemical doesn’t break down quickly, so it ends up moving through soil and water if not handled properly.
Industrial plants can cut these risks by using closed systems, recycling streams that catch stray molecules, and thoroughly training workers about handling acids safely. I’ve seen cases where simple changes—better valves, improved storage—cut emissions down to almost nothing. Monitoring wastewater and air for traces, rather than waiting for problems, also keeps things safer for communities nearby.
No single company can solve the waste problem alone. Collaboration with universities and regulatory agencies opens up new, greener processes—finding catalysts that need less acidic input or break down leftover chemicals. Efforts to engineer plants and microbes that eat up these compounds could shift practices in a decade or less. When I look at trends across Europe and North America, the push for safer substitutes grows each year, and that pressure sparks real innovation. Many in the industry already know: paying attention to small molecules like 2-ethylhexanoic acid leads to cleaner water, safer workplaces, and better health down the line.
Few folks outside the chemical industry have heard the name 2-Ethylhexanoic Acid, but this clear, oily liquid shows up in paints, coolants, lubricants, and even plastics. Many workplaces bring people into daily contact with it, and it finds its way into wastewater from factories. The general public rarely sees it, though employees in chemical plants and packaging sectors might not get the same comfort.
Scientists and safety organizations haven’t always spoken in one clear voice about the dangers of this acid. The European Chemicals Agency classifies it as ‘hazardous’—not a word they throw around lightly. The reasons for this status rest in real-life lab results: studies suggest it can irritate the eyes and skin pretty harshly. Breathing in high amounts can mess with a person’s respiratory system. Lab animals exposed to large doses saw damage in organs such as their kidneys and liver. Researchers worry about developmental toxicity too, since pregnant animals sometimes gave birth to offspring with health problems after exposure.
Beyond lab tests, the workplace experience matters. According to OSHA records and the National Institute for Occupational Safety and Health, regular handling without protection creates risks. I’ve watched colleagues neglect goggles or gloves because the clear liquid “doesn’t seem so bad.” Red hands, watery eyes, and nasty coughs happened more often than people would like to admit. Most folks want to believe that something used in industry across the globe can’t possibly be that toxic, but experience—and the science—say otherwise.
Industry often downplays small-scale exposure. In many shops, workers get brief training and a stack of safety sheets, yet pressure to finish the job fast trumps careful handling. That leads to splashes, leaks, and contact with skin. Some studies link chronic low-level exposure to problems in the liver or blood chemistry. Since not every country tracks its chemical-related illnesses the same way, plenty of health impacts go unreported or fall through the cracks.
Runoff and improper disposal raise another layer of concern. Once 2-Ethylhexanoic Acid makes it to rivers or waste treatment plants, fish and other wildlife pick up doses big enough to hurt their kidneys or reproduction. Water purification usually takes care of most industrial chemicals, but sometimes this acid slides through in low concentrations, adding up over time. Cities depending on vulnerable water sources should take this threat seriously—especially rural areas downstream from big plants.
Safe handling cuts risks right away. Workers need better access to gloves, goggles, and proper uniforms—employers should give actual training, walking people through what the chemical does, what symptoms to watch for, and how to react if something goes wrong. Facilities must watch their wastewater, keeping levels well below legal limits and repairing leaks before they add up to bigger problems. Clear labeling, regular health checkups for employees, and honest conversations can change the story.
Despite warnings and reports, people tend to let their guard down when nothing bad happens right away. That delay between exposure and symptoms means the true hazards of 2-Ethylhexanoic Acid can go overlooked. The challenge isn’t only figuring out how toxic it really is, but building habits and systems where people stay safe without shortcuts. Chemists, factory leads, and workers all have a role here—because waiting until someone gets hurt isn’t good enough.
Anyone working in a facility where 2-Ethylhexanoic Acid shows up knows the stuff carries a sting. The odor scratches at your nose, and a splash on unprotected skin sends a message right away—pay attention. Most chemical workers have faced days when a mishap turns routine storage into a health hazard. It’s not just about ticking off checklists; it’s about keeping each other healthy and keeping operations humming without a hitch.
Take a look at the facts. 2-Ethylhexanoic Acid can irritate skin and eyes. Breathing in the vapors or dust puts airways at risk, especially indoors. Long-term exposure has sparked plenty of research, with some studies showing liver and kidney effects in lab animals at high doses. The risk ramps up when the basics get skipped—open drums, poor ventilation, ignored labels. That’s more than just an inconvenience; that could mean lost days, hospital visits, investigations, and damage to a company’s reputation.
I’ve seen some folks store acids in whatever space they can find, even next to flammable materials or food items. That sort of shortcut invites disaster. The best practice is to keep 2-Ethylhexanoic Acid in a cool, well-ventilated place, far from sunlight and sources of ignition. Dedicated acid storage cabinets with corrosion-resistant shelves and doors lock out accidents. Leaks don’t seep onto the floor if secondary containment trays catch the spill. Storing containers upright and tight-fitting cap keeps the vapors safely bottled up.
Full-face shields, chemical-resistant gloves, and long sleeves make a real difference. Many workers get tempted to duck out on full gear, especially if the shift is hot and the job looks quick. I've seen burns and rashes pop up among people rushing the job wearing short sleeves. The right gear matters every time, no matter how small the task seems.
Opening containers should happen only in areas with strong local exhaust or fume hoods. Reliable equipment catches the invisible mist before it can reach your lungs. Not every workplace puts an eyewash station and safety shower within reach. Consider that a mistake. If an accident happens, fast access limits injury and shows workers you respect their wellbeing.
Labels fade and go missing in the chaos of a busy shift. Legible, durable labeling keeps the next crew in the loop and stops mix-ups before they start. In one plant, mixing up chemicals led to a runaway reaction that forced an evacuation. Clear communication avoids these costly slip-ups. Training everyone—new hires, temp workers, veterans—creates muscle memory. Practice drills, walk-throughs, and refreshers keep awareness sharp.
Disposal counts too. Dumping leftovers down the drain isn’t just illegal; it poisons waterways and brings hefty fines. Licensed hazardous waste handlers should deal with the waste, and tracking paperwork covers your back if the authorities come knocking.
Some companies still skimp on safety to save a buck. The better approach invests in small upgrades—better personal protective equipment, frequent refresher sessions, tight-topped acid cabinets. It doesn’t take a PhD to notice the payoff. Injuries drop, morale rises, and the business earns trust.
Handling 2-Ethylhexanoic Acid takes respect, not just rules. A good system means everyone walks out healthy at the end of the day. That’s how a strong crew keeps each other safe and the work on track.
Every day, paint and plastic manufacturers reach for metal soaps, and 2-ethylhexanoic acid makes those possible. When you think about glossy paint on a new car or that smooth plastic on everyday gadgets, this acid has usually played a part. Metal soaps made from it, like zinc and cobalt salts, help paint cure faster and harder, which matters when a manufacturer faces tight deadlines and expects long-lasting results. In plastics, these metal soaps keep things flexible and stable. It’s this reliability that sets products apart on crowded store shelves.
The average car on the road works under harsh conditions—changing temperatures, relentless friction, and unpredictable weather. To protect engines and parts from corrosion, many coolant and lubricant makers choose this acid. It’s not about hype. 2-ethylhexanoic acid reacts well with other additives, holding up in tough environments, which means less risk of breakdown and expensive repairs. Research from automotive chemistry shows these additives extend the life of both coolants and lubricants, reducing waste and lowering mechanical failure rates. People who drive older vehicles especially benefit from these enhanced fluids, stretching every mile further.
Factories depend on machinery that runs for hours and sometimes days on end. Here, plasticizers made with 2-ethylhexanoic acid keep moving parts smooth and production lines humming. Synthetic lubricants using this acid resist high temperatures and heavy loads, letting these machines work without constant downtime for oil changes. Plant managers watch their bottom line, and chemicals that boost performance without raising maintenance costs deserve attention. Industry studies back this up: these additives handle heat cycles and high pressure, giving facilities a reliable way to meet targets without unexpected hiccups.
No one likes streaky or faded coatings, whether it’s paint on a home or industrial protective layers on equipment. 2-ethylhexanoic acid finds a home in pigment dispersants, helping pigments spread more evenly. That means colors stay true longer. In my work with contractors, the difference between a quality finish and a patchy mess often comes down to what’s in the can—these acid-based dispersants deliver that consistency. It's a simple fix to a common headache in surface finishing industries.
Nothing useful comes without trade-offs. The toxicity concerns around 2-ethylhexanoic acid—especially its impact on aquatic life and workplace safety—push companies to rethink how much they use and how they dispose of waste. Regulators have stepped up oversight, and more companies track their chemical footprints closely. Experience from other fields shows that switching to safer alternatives or closed-loop recycling systems pays off. In chemistry labs, newer green additives enter the market all the time, but matching the effectiveness and versatility of this acid still takes work, planning, and investment.
2-ethylhexanoic acid serves as more than a simple ingredient. It shapes outcomes, feeds progress in manufacturing, and keeps core products running longer. Companies that value durability, cost savings, and environmental care spend real time understanding the full range of its uses—and look for ways to work smarter with it or replace it responsibly. Real change happens through clear facts, honest review, and a commitment to practical improvements across every stage of production.
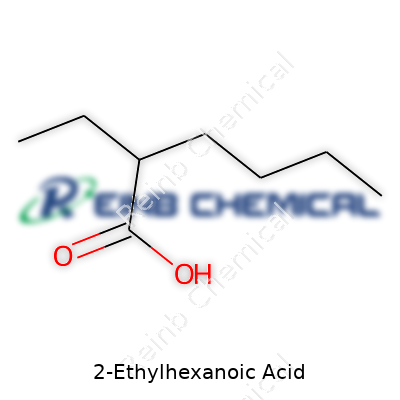
| Names | |
| Preferred IUPAC name | 2-Ethylhexanoic acid |
| Other names |
2-Ethylcaproic acid 2-Ethylhexanoate Octanoic acid, 2-ethyl- 2-Ethylhexoic acid |
| Pronunciation | /tuː ˌɛθ.ɪl.hɛkˈsæ.nəʊ.ɪk ˈæs.ɪd/ |
| Identifiers | |
| CAS Number | 149-57-5 |
| Beilstein Reference | 1718732 |
| ChEBI | CHEBI:30853 |
| ChEMBL | CHEMBL11541 |
| ChemSpider | 6964 |
| DrugBank | DB04248 |
| ECHA InfoCard | DTXSID5020735 |
| EC Number | 211-047-3 |
| Gmelin Reference | 8597 |
| KEGG | C01829 |
| MeSH | D017811 |
| PubChem CID | 7991 |
| RTECS number | AT4900000 |
| UNII | 3K9958V90M |
| UN number | UN2810 |
| Properties | |
| Chemical formula | C8H16O2 |
| Molar mass | 144.21 g/mol |
| Appearance | Clear, colorless to pale yellow liquid. |
| Odor | slight characteristic |
| Density | 0.904 g/cm3 |
| Solubility in water | slightly soluble |
| log P | 2.7 |
| Vapor pressure | 0.022 hPa (20 °C) |
| Acidity (pKa) | 4.89 |
| Basicity (pKb) | 7.6 |
| Magnetic susceptibility (χ) | -51.0e-6 cm³/mol |
| Refractive index (nD) | 1.423 |
| Viscosity | 14.2 mPa·s (25°C) |
| Dipole moment | 1.93 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 359.60 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -471.9 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -3947.2 kJ/mol |
| Pharmacology | |
| ATC code | JECFA Non-ATC |
| Hazards | |
| Main hazards | Harmful if swallowed, causes serious eye irritation, may cause damage to organs through prolonged or repeated exposure. |
| GHS labelling | GHS07, GHS08 |
| Pictograms | GHS05,GHS07 |
| Signal word | Warning |
| Hazard statements | H315, H319, H361d |
| Precautionary statements | P264, P270, P273, P280, P301+P312, P305+P351+P338, P330, P337+P313, P501 |
| NFPA 704 (fire diamond) | 2-1-0 |
| Flash point | 107 °C |
| Autoignition temperature | 430 °C (806 °F; 703 K) |
| Explosive limits | Explosive limits: 0.9–7% |
| Lethal dose or concentration | LD50 (oral, rat): 2043 mg/kg |
| LD50 (median dose) | LD50 (median dose): Rat oral 2043 mg/kg |
| NIOSH | NA6895000 |
| PEL (Permissible) | PEL (Permissible Exposure Limit) for 2-Ethylhexanoic Acid: "5 mg/m³ (inhalable fraction and vapor) as an 8-hour TWA (ACGIH TLV) |
| REL (Recommended) | 5 mg/m³ |
| IDLH (Immediate danger) | IDLH: 100 ppm |
| Related compounds | |
| Related compounds |
Caprylic acid Valproic acid Isobutyric acid 2-Ethylhexanol Octanoic acid |